Page 210 - Withrow and MacEwen's Small Animal Clinical Oncology, 6th Edition
P. 210
CHAPTER 12 Cancer Chemotherapy 189
1000 C max 10,000
VetBooks.ir 8,000
6,000
100 Neutrophil nadir (cells/ L) 4,000
[Drug] plasma (ng/mL) 10 A 0 0.0 1.0 AUC (min • mg • mL 1 ) 4.0 5.0
2,000
2.0
3.0
AUC 15
1 10
0 4 8 12 Cl pt (mL • min 1 • kg 1 )
Time (hr)
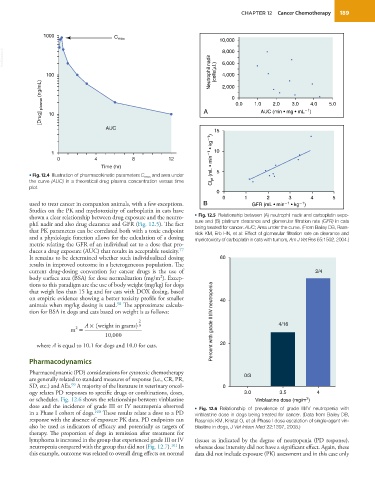
• Fig. 12.4 Illustration of pharmacokinetic parameters C max and area under 5
the curve (AUC) in a theoretical drug plasma concentration versus time
plot.
0
0 1 2 3 4 5
used to treat cancer in companion animals, with a few exceptions. B GFR (mL • min 1 • kg 1 )
Studies on the PK and myelotoxicity of carboplatin in cats have
shown a clear relationship between drug exposure and the neutro- • Fig. 12.5 Relationship between (A) neutrophil nadir and carboplatin expo-
phil nadir and also drug clearance and GFR (Fig. 12.5). The fact sure and (B) platinum clearance and glomerular filtration rate (GFR) in cats
that PK parameters can be correlated both with a toxic endpoint being treated for cancer. AUC; Area under the curve. (From Bailey DB, Rass-
and a physiologic function allows for the calculation of a dosing nick KM, Erb HN, et al: Effect of glomerular filtration rate on clearance and
myelotoxicity of carboplatin in cats with tumors, Am J Vet Res 65:1502, 2004.)
metric relating the GFR of an individual cat to a dose that pro-
77
duces a drug exposure (AUC) that results in acceptable toxicity.
It remains to be determined whether such individualized dosing 60
results in improved outcome in a heterogeneous population. The
current drug-dosing convention for cancer drugs is the use of 2/4
body surface area (BSA) for dose normalization (mg/m ). Excep-
2
tions to this paradigm are the use of body weight (mg/kg) for dogs
that weigh less than 15 kg and for cats with DOX dosing, based
on empiric evidence showing a better toxicity profile for smaller 40
animals when mg/kg dosing is used. The approximate calcula-
98
tion for BSA in dogs and cats based on weight is as follows:
2 Percent with grade III/IV neutropenia 4/16
2 A × (weight in grams) 3
m =
10,000
where A is equal to 10.1 for dogs and 10.0 for cats. 20
Pharmacodynamics
Pharmacodynamic (PD) considerations for cytotoxic chemotherapy
are generally related to standard measures of response (i.e., CR, PR, 0/3
SD, etc.) and AEs. A majority of the literature in veterinary oncol- 0
99
ogy relates PD responses to specific drugs or combinations, doses, 3.0 3.5 4
or schedules. Fig. 12.6 shows the relationships between vinblastine Vinblastine dose (mg/m )
2
dose and the incidence of grade III or IV neutropenia observed • Fig. 12.6 Relationship of prevalence of grade III/IV neutropenia with
in a Phase I cohort of dogs. 100 These results relate a dose to a PD vinblastine dose in dogs being treated for cancer. (Data from Bailey DB,
response with the absence of exposure PK data. PD endpoints can Rassnick KM, Kristal O, et al: Phase I dose escalation of single-agent vin-
also be used as indicators of efficacy and potentially as targets of blastine in dogs, J Vet Intern Med 22:1397, 2008.)
therapy. The proportion of dogs in remission after treatment for
lymphoma is increased in the group that experienced grade III or IV tissues as indicated by the degree of neutropenia (PD response),
neutropenia compared with the group that did not (Fig. 12.7). 101 In whereas dose intensity did not have a significant effect. Again, these
this example, outcome was related to overall drug effects on normal data did not include exposure (PK) assessment and in this case only