Page 262 - Withrow and MacEwen's Small Animal Clinical Oncology, 6th Edition
P. 262
CHAPTER 14 Cancer Immunotherapy 241
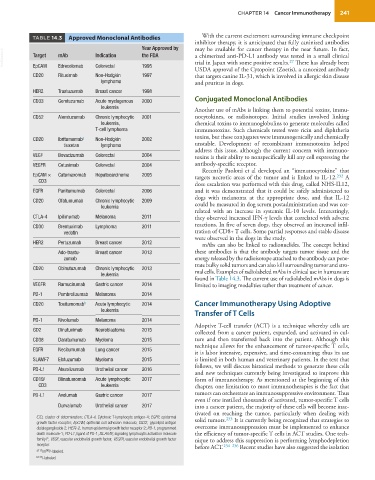
TABLE 14.3 Approved Monoclonal Antibodies With the current excitement surrounding immune checkpoint
inhibitor therapy, it is anticipated that fully caninized antibodies
Year Approved by may be available for cancer therapy in the near future. In fact,
VetBooks.ir Target mAb Indication the FDA a chimerized anti-PD-L1 antibody was tested in a small clinical
27
trial in Japan with some positive results. There has already been
1995
EpCAM
Edrecolomab
Colorectal
USDA approval of the Cytopoint (Zoetis), a canonized antibody
CD20 Rituximab Non-Hodgkin 1997 that targets canine IL-31, which is involved in allergic skin disease
lymphoma and pruritus in dogs.
HER2 Trastuzumab Breast cancer 1998
Conjugated Monoclonal Antibodies
CD33 Gemtuzumab Acute myelogenous 2000
leukemia Another use of mAbs is linking them to potential toxins, immu-
CD52 Alemtuzumab Chronic lymphocytic 2001 nocytokines, or radioisotopes. Initial studies involved linking
leukemia, chemical toxins to immunoglobulins to generate molecules called
T-cell lymphoma immunotoxins. Such chemicals tested were ricin and diphtheria
toxins, but these conjugates were immunogenically and chemically
CD20 Ibritumomab Non-Hodgkin 2002
a
tiuxetan lymphoma unstable. Development of recombinant immunotoxins helped
address this issue, although the current concern with immuno-
VEGF Bevacizumab Colorectal 2004 toxins is their ability to nonspecifically kill any cell expressing the
VEGFR Cetuximab Colorectal 2004 antibody-specific receptor.
Recently Paoloni et al developed an “immunocytokine” that
EpCAM × Catumaxomab Hepatocarcinoma 2005 targets necrotic areas of the tumor and is linked to IL-12. 232 A
CD3
dose escalation was performed with this drug, called NHS-IL12,
EGFR Panitumumab Colorectal 2006 and it was demonstrated that it could be safely administered to
dogs with melanoma at the appropriate dose, and that IL-12
CD20 Ofatumumab Chronic lymphocytic 2009 could be measured in dog serum postadministration and was cor-
leukemia
related with an increase in systemic IL-10 levels. Interestingly,
CTLA-4 Ipilimumab Melanoma 2011 they observed increased IFN-γ levels that correlated with adverse
CD30 Brentuximab Lymphoma 2011 reactions. In five of seven dogs, they observed an increased infil-
vedotin tration of CD8+ T cells. Some partial responses and stable disease
were observed in the dogs in the study.
HER2 Pertuzumab Breast cancer 2012 mAbs can also be linked to radionuclides. The concept behind
Ado-trastu- Breast cancer 2013 these antibodies is that the antibody targets tumor tissue and the
zumab energy released by the radioisotope attached to the antibody can pene-
trate bulky solid tumors and can also kill surrounding tumor and stro-
CD20 Obinutuzumab Chronic lymphocytic 2013 mal cells. Examples of radiolabeled mAbs in clinical use in humans are
leukemia
found in Table 14.3. The current use of radiolabeled mAbs in dogs is
VEGFR Ramucirumab Gastric cancer 2014 limited to imaging modalities rather than treatment of cancer.
PD-1 Pembrolizumab Melanoma 2014
CD20 Tositumomab b Acute lymphocytic 2014 Cancer Immunotherapy Using Adoptive
leukemia Transfer of T Cells
PD-1 Nivolumab Melanoma 2014
Adoptive T-cell transfer (ACT) is a technique whereby cells are
GD2 Dinutuximab Neuroblastoma 2015 collected from a cancer patient, expanded, and activated in cul-
CD38 Daratumumab Myeloma 2015 ture and then transferred back into the patient. Although this
technique allows for the enhancement of tumor-specific T cells,
EGFR Necitumumab Lung cancer 2015
it is labor intensive, expensive, and time-consuming; thus its use
SLAMF7 Elotuzumab Myeloma 2015 is limited in both human and veterinary patients. In the text that
follows, we will discuss historical methods to generate these cells
PD-L1 Atezolizumab Urothelial cancer 2016
and new techniques currently being investigated to improve this
CD19/ Blinatumomab Acute lymphocytic 2017 form of immunotherapy. As mentioned at the beginning of this
CD3 leukemia chapter, one limitation to most immunotherapies is the fact that
PD-L1 Avelumab Gastric cancer 2017 tumors can orchestrate an immunosuppressive environment. Thus
even if one instilled thousands of activated, tumor-specific T cells
Durvalumab Urothelial cancer 2017 into a cancer patient, the majority of these cells will become inac-
tivated on reaching the tumor, particularly when dealing with
CD, cluster of determination; CTLA-4, Cytotoxic T-lymphocyte antigen-4; EGFR, epidermal solid tumors. 233 It is currently being recognized that strategies to
growth factor receptor; EpCAM, epithelial cell adhesion molecule; GD2, glycolipid antigen
disialoganglioside 2; HER-2, human epidermal growth factor receptor 2; PD-1, programmed overcome immunosuppression must be implemented to enhance
death molecule-1; PD-L1, ligand of PD-1; SLAMF, signaling lymphocytic activation molecule the efficiency of tumor-specific T cells in ACT studies. One tech-
family F; VEGF, vascular endothelial growth factor; VEGFR, vascular endothelial growth factor nique to address this suppression is performing lymphodepletion
receptor. before ACT. 234–236 Recent studies have also suggested the isolation
a111 In/ Y-labeled.
90
b131 I-labeled.