Page 1105 - Veterinary Immunology, 10th Edition
P. 1105
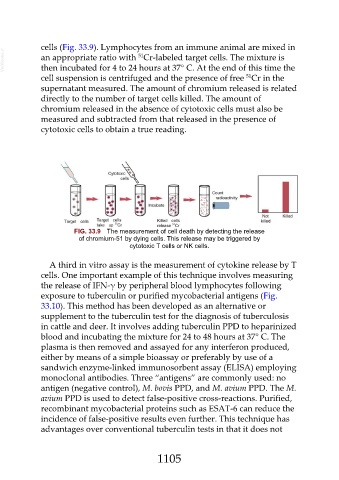
cells (Fig. 33.9). Lymphocytes from an immune animal are mixed in
VetBooks.ir an appropriate ratio with Cr-labeled target cells. The mixture is
51
then incubated for 4 to 24 hours at 37° C. At the end of this time the
51
cell suspension is centrifuged and the presence of free Cr in the
supernatant measured. The amount of chromium released is related
directly to the number of target cells killed. The amount of
chromium released in the absence of cytotoxic cells must also be
measured and subtracted from that released in the presence of
cytotoxic cells to obtain a true reading.
FIG. 33.9 The measurement of cell death by detecting the release
of chromium-51 by dying cells. This release may be triggered by
cytotoxic T cells or NK cells.
A third in vitro assay is the measurement of cytokine release by T
cells. One important example of this technique involves measuring
the release of IFN-γ by peripheral blood lymphocytes following
exposure to tuberculin or purified mycobacterial antigens (Fig.
33.10). This method has been developed as an alternative or
supplement to the tuberculin test for the diagnosis of tuberculosis
in cattle and deer. It involves adding tuberculin PPD to heparinized
blood and incubating the mixture for 24 to 48 hours at 37° C. The
plasma is then removed and assayed for any interferon produced,
either by means of a simple bioassay or preferably by use of a
sandwich enzyme-linked immunosorbent assay (ELISA) employing
monoclonal antibodies. Three “antigens” are commonly used: no
antigen (negative control), M. bovis PPD, and M. avium PPD. The M.
avium PPD is used to detect false-positive cross-reactions. Purified,
recombinant mycobacterial proteins such as ESAT-6 can reduce the
incidence of false-positive results even further. This technique has
advantages over conventional tuberculin tests in that it does not
1105