Page 231 - Natural Antioxidants, Applications in Foods of Animal Origin
P. 231
210 Natural Antioxidants: Applications in Foods of Animal Origin
VetBooks.ir in electrochemistry, that is the development of the understanding of the rela-
tionship between the amount of an electrical charge and the quantity of a
chemical compound, led to improvements in faster, more precise analysis
of hydroperoxides in meat samples. Electrochemical methods quantify
compounds produced from lipid oxidation potentiometrically.
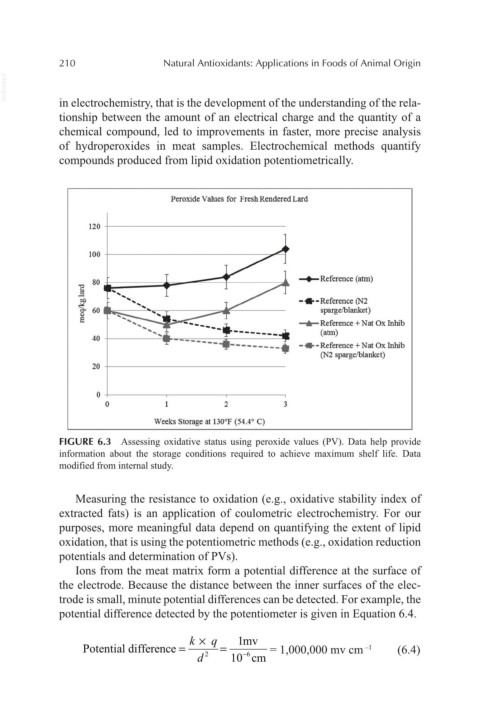
Peroxide Values for Fresh Rendered Lard
120
100
.., 80 ~Reference (atm)
ll
t! -•-Reference (N2
0" 60 sparge/blanket)
" .....,._Reference +Nat Ox Inhib
E
(atm)
40
- •-Reference +Nat Ox Inhib
(N2 sparge/blanket)
20
0
0 2
Weeks Storage at 130°F (54.4° C)
FIGURE 6.3 Assessing oxidative status using peroxide values (PV). Data help provide
information about the storage conditions required to achieve maximum shelf life. Data
modified from internal study.
Measuring the resistance to oxidation (e.g., oxidative stability index of
extracted fats) is an application of coulometric electrochemistry. For our
purposes, more meaningful data depend on quantifying the extent of lipid
oxidation, that is using the potentiometric methods (e.g., oxidation reduction
potentials and determination of PVs).
Ions from the meat matrix form a potential difference at the surface of
the electrode. Because the distance between the inner surfaces of the elec-
trode is small, minute potential differences can be detected. For example, the
potential difference detected by the potentiometer is given in Equation 6.4.
q
k × 1mv
Potential difference = = = 1,000,000 mv cm –1 (6.4)
d 2 1 0 cm
−
6