Page 232 - Natural Antioxidants, Applications in Foods of Animal Origin
P. 232
Methods and Their Applications for Measuring 211
VetBooks.ir where k is a constant,
mv = millivolt,
q is magnitude of the interfacial electrical potential developed between the
meat sample and electrode,
d is square of the interfacial distance between the meat and electrode.
2
The electrochemical amplification of the potential offers the sensi-
tivity needed when the detection of small differences is needed. This
method would be considered when the background color of the sample or
the concentration of the analyte is low (limit of detection (LOD) = 0.01 M
and LOQ = 0.16 meq. Kg ). Potentiometric determination of hydroperox-
–1
ides is based on Equation 6.5, an application of the Nernst principles for
electrochemistry.
0.059 [reduced ]
E
=
E o − log (6.5)
n 10 [oxidized ]
Application of the Nernst equation relates the electrical potential to
aqueous concentrations of a solution under controlled conditions (Karddash-
Strochkova & Tur’ yan, 2001). The analytical instrumentation is similar
to the method for measuring PVs with potassium iodide–starch reagents.
Although both are based on titration, the potentiometric method uses a
change in an electrical signal whereas the iodometric method depends on a
visual color change to determine the concentration of the hydroperoxides in
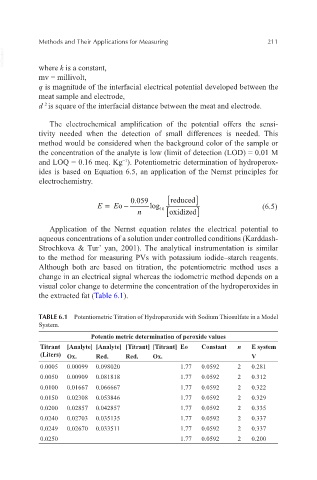
the extracted fat (Table 6.1).
TABLE 6.1 Potentiometric Titration of Hydroperoxide with Sodium Thiosulfate in a Model
System.
Potentio metric determination of peroxide values
Titrant [Analyte] [Analyte] [Titrant] [Titrant] Eo Constant n E system
(Liters) Ox. Red. Red. Ox. V
0.0005 0.00099 0.098020 1.77 0.0592 2 0.281
0.0050 0.00909 0.081818 1.77 0.0592 2 0.312
0.0100 0.01667 0.066667 1.77 0.0592 2 0.322
0.0150 0.02308 0.053846 1.77 0.0592 2 0.329
0.0200 0.02857 0.042857 1.77 0.0592 2 0.335
0.0240 0.02703 0.035135 1.77 0.0592 2 0.337
0.0249 0.02670 0.033511 1.77 0.0592 2 0.337
0.0250 1.77 0.0592 2 0.200