Page 626 - Clinical Small Animal Internal Medicine
P. 626
594 Section 6 Gastrointestinal Disease
(a) assay (SNAP‐cPL™, Idexx Laboratories) that shows good
VetBooks.ir alignment and reproducibility with the laboratory‐based
ELISA.
There have been a number of studies evaluating cPL
for the diagnosis of pancreatitis (Table 55.2). Overall,
cPL should be considered highly specific for pancreatitis.
Sensitivity of the cPL assay appears to be high in animals
with AP, but less so for CP. When interpreting cPL, it
should be remembered that pancreatic inflammation
may be present, but not be the cause of the clinical pres-
entation. The most relevant application of this is when
there are duodenal foreign bodies or septic peritonitis
that can cause “bystander” pancreatic inflammation.
(b) Misdiagnosis in this situation could fail to identify that
surgical correction is required.
Pancreatic elastase (PE‐1) is another enzyme consid-
ered to be pancreas specific. There is a commercially
.
available canine PE‐1 (cPE‐1) ELISA (ScheBo Elastase
1‐Canine, ScheBo Biotech AG, Giessen, Germany). It
appears that PE‐1 has wide variation in healthy dogs, and
similar sensitivity and specificity to cPL when assessing
dogs with severe AP (see Table 55.2). However, this
premise should be tested in a larger cohort of dogs.
Diagnostic Imaging
Diagnostic imaging is an important component of the
investigation of dogs presenting with signs of abdominal
disease. The major indication for abdominal radiographs
is to rule out the presence of surgical disease such as
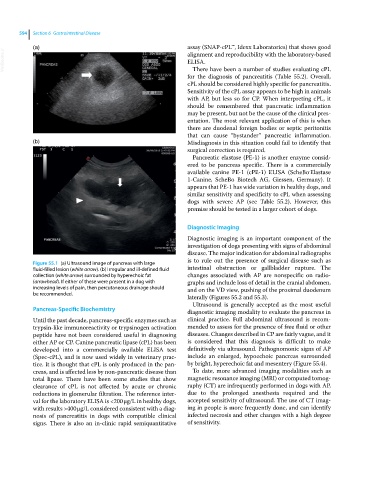
Figure 55.1 (a) Ultrasound image of pancreas with large
fluid‐filled lesion (white arrow). (b) Irregular and ill‐defined fluid intestinal obstruction or gallbladder rupture. The
collection (white arrow) surrounded by hyperechoic fat changes associated with AP are nonspecific on radio-
(arrowhead). If either of these were present in a dog with graphs and include loss of detail in the cranial abdomen,
increasing levels of pain, then percutaneous drainage should and on the VD view, pushing of the proximal duodenum
be recommended.
laterally (Figures 55.2 and 55.3).
Ultrasound is generally accepted as the most useful
Pancreas‐Specific Biochemistry diagnostic imaging modality to evaluate the pancreas in
Until the past decade, pancreas‐specific enzymes such as clinical practice. Full abdominal ultrasound is recom-
trypsin‐like immunoreactivity or trypsinogen activation mended to assess for the presence of free fluid or other
peptide have not been considered useful in diagnosing diseases. Changes described in CP are fairly vague, and it
either AP or CP. Canine pancreatic lipase (cPL) has been is considered that this diagnosis is difficult to make
developed into a commercially available ELISA test definitively via ultrasound. Pathognomonic signs of AP
(Spec‐cPL), and is now used widely in veterinary prac- include an enlarged, hypoechoic pancreas surrounded
tice. It is thought that cPL is only produced in the pan- by bright, hyperechoic fat and mesentery (Figure 55.4).
creas, and is affected less by non-pancreatic disease than To date, more advanced imaging modalities such as
total lipase. There have been some studies that show magnetic resonance imaging (MRI) or computed tomog-
clearance of cPL is not affected by acute or chronic raphy (CT) are infrequently performed in dogs with AP,
reductions in glomerular filtration. The reference inter- due to the prolonged anesthesia required and the
val for the laboratory ELISA is <200 μg/L in healthy dogs, accepted sensitivity of ultrasound. The use of CT imag-
with results >400 μg/L considered consistent with a diag- ing in people is more frequently done, and can identify
nosis of pancreatitis in dogs with compatible clinical infected necrosis and other changes with a high degree
signs. There is also an in‐clinic rapid semiquantitative of sensitivity.