Page 161 - Medicinal Chemistry Self Assessment
P. 161
150 Medicinal Chemistry Self Assessment
Answer
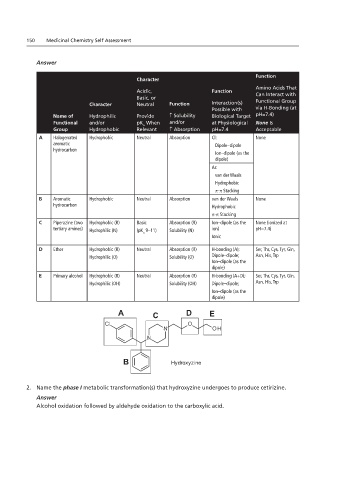
Function
Character
Amino Acids That
Acidic, Function Can Interact with
Basic, or
Character Neutral Function Interaction(s) Functional Group
Possible with via H-Bonding (at
Name of Hydrophilic Provide ↑ Solubility Biological Target pH=7.4)
Functional and/or pK When and/or at Physiological None Is
a
Group Hydrophobic Relevant ↑ Absorption pH=7.4 Acceptable
A Halogenated Hydrophobic Neutral Absorption Cl: None
aromatic Dipole–dipole
hydrocarbon
Ion–dipole (as the
Medicinal Chemistry Self-Assessment Book: Batch Two
dipole)
Chapters 1.11 and 2.11
Ar:
van der Waals
Chapter 1.11 (remove bolded drug names)
Hydrophobic
π-π Stacking
B Aromatic Hydrophobic Neutral Absorption van der Waals None
hydrocarbon Hydrophobic
π-π Stacking
C Piperazine (two Hydrophobic (R) Basic Absorption (R) Ion–dipole (as the None (ionized at
tertiary amines) Hydrophilic (N) (pK 9–11) Solubility (N) ion) pH=7.4)
a
Ionic
D Ether Hydrophobic (R) Neutral Absorption (R) H-bonding (A); Ser, Thr, Cys, Tyr, Gln,
Cetirizine
Hydrophilic (O) Hydroxyzine Solubility (O) Dipole–dipole; Asn, His, Trp
Ion–dipole (as the
dipole)
E Primary alcohol Hydrophobic (R) Neutral Absorption (R) H-bonding (A+D); Ser, Thr, Cys, Tyr, Gln,
Hydrophilic (OH) Solubility (OH) Dipole–dipole; Asn, His, Trp
Chapter 2.11 (remove bolded drug names) Ion–dipole (as the
dipole)
A C D E
B Hydroxyzine
2. Name the phase I metabolic transformation(s) that hydroxyzine undergoes to produce cetirizine.
Answer
Alcohol oxidation followed by aldehyde oxidation to the carboxylic acid.