Page 156 - Medicinal Chemistry Self Assessment
P. 156
2.10 Cefprozil 145
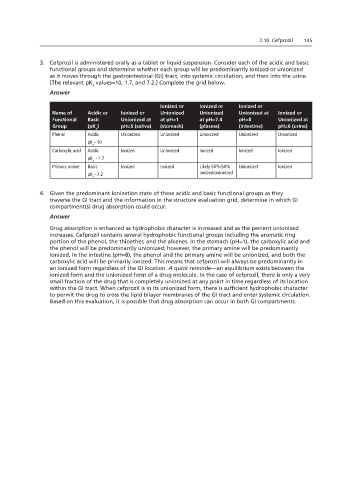
3. Cefprozil is administered orally as a tablet or liquid suspension. Consider each of the acidic and basic
functional groups and determine whether each group will be predominantly ionized or unionized
as it moves through the gastrointestinal (GI) tract, into systemic circulation, and then into the urine.
[The relevant pK values=10, 1.7, and 7.2.] Complete the grid below.
a
Answer
Ionized or Ionized or Ionized or
Name of Acidic or Ionized or Unionized Unionized Unionized at Ionized or
Functional Basic Unionized at at pH=1 at pH=7.4 pH=8 Unionized at
Group (pK ) pH=5 (saliva) (stomach) (plasma) (intestine) pH=6 (urine)
a
Phenol Acidic Unionized Unionized Unionized Unionized Unionized
pK ~10
a
Carboxylic acid Acidic Ionized Unionized Ionized Ionized Ionized
pK ~1.7
a
Primary amine Basic Ionized Ionized Likely 50%/50% Unionized Ionized
pK ~7.2 ionized/unionized
a
4. Given the predominant ionization state of these acidic and basic functional groups as they
traverse the GI tract and the information in the structure evaluation grid, determine in which GI
compartment(s) drug absorption could occur.
Answer
Drug absorption is enhanced as hydrophobic character is increased and as the percent unionized
increases. Cefprozil contains several hydrophobic functional groups including the aromatic ring
portion of the phenol, the thioether, and the alkenes. In the stomach (pH=1), the carboxylic acid and
the phenol will be predominantly unionized; however, the primary amine will be predominantly
ionized. In the intestine (pH=8), the phenol and the primary amine will be unionized, and both the
carboxylic acid will be primarily ionized. This means that cefprozil will always be predominantly in
an ionized form regardless of the GI location. A quick reminde—an equilibrium exists between the
ionized form and the unionized form of a drug molecule. In the case of cefprozil, there is only a very
small fraction of the drug that is completely unionized at any point in time regardless of its location
within the GI tract. When cefprozil is in its unionized form, there is sufficient hydrophobic character
to permit the drug to cross the lipid bilayer membranes of the GI tract and enter systemic circulation.
Based on this evaluation, it is possible that drug absorption can occur in both GI compartments.