Page 151 - Medicinal Chemistry Self Assessment
P. 151
140 Medicinal Chemistry Self Assessment
Answer
Answer
H
O N R 4
Gln
R 3
H
N
O Asp O H
R 1 H Hydrogen Bond
N
R 2 H O
O N
Ionic Bond
– O
Cl Cl O
N N +
van der Waals; H H van der Waals;
Hydrophobic Interaction
N
Hydrophobic Interaction
R 6 R 5
R 8 OH O
N Ile
H
R 7
O Tyr
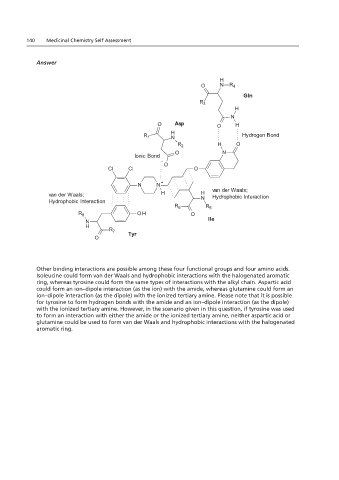
Other binding interactions are possible among these four functional groups and four amino acids.
Isoleucine could form van der Waals and hydrophobic interactions with the halogenated aromatic
ring, whereas tyrosine could form the same types of interactions with the alkyl chain. Aspartic acid
could form an ion–dipole interaction (as the ion) with the amide, whereas glutamine could form an
ion–dipole interaction (as the dipole) with the ionized tertiary amine. Please note that it is possible
for tyrosine to form hydrogen bonds with the amide and an ion–dipole interaction (as the dipole)
with the ionized tertiary amine. However, in the scenario given in this question, if tyrosine was used
to form an interaction with either the amide or the ionized tertiary amine, neither aspartic acid or
glutamine could be used to form van der Waals and hydrophobic interactions with the halogenated
aromatic ring.