Page 155 - Medicinal Chemistry Self Assessment
P. 155
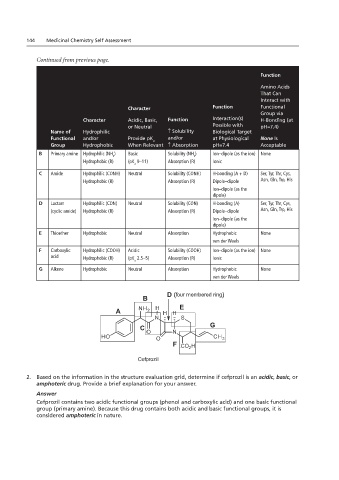
144 Medicinal Chemistry Self Assessment
Continued from previous page.
Function
Amino Acids
That Can
Interact with
Character Function Functional
Group via
Character Acidic, Basic, Function Interaction(s) H-Bonding (at
or Neutral Possible with pH=7.4)
Name of Hydrophilic ↑ Solubility Biological Target
Functional and/or Provide pK and/or at Physiological None Is
a
Group Hydrophobic When Relevant ↑ Absorption pH=7.4 Acceptable
B Primary amine Hydrophilic (NH ) Basic Solubility (NH ) Ion–dipole (as the ion) None
2 2
Hydrophobic (R) (pK 9–11) Absorption (R) Ionic
a
C Amide Hydrophilic (CONH) Neutral Solubility (CONH) H-bonding (A + D) Ser, Tyr, Thr, Cys,
Hydrophobic (R) Absorption (R) Dipole–dipole Asn, Gln, Trp, His
Ion–dipole (as the
dipole)
D Lactam Hydrophilic (CON) Neutral Solubility (CON) H-bonding (A) Ser, Tyr, Thr, Cys,
(cyclic amide) Hydrophobic (R) Absorption (R) Dipole–dipole Asn, Gln, Trp, His
Ion–dipole (as the
dipole)
E Thioether Hydrophobic Neutral Absorption Hydrophobic None
van der Waals
F Carboxylic Hydrophilic (COOH) Acidic Solubility (COOH) Ion–dipole (as the ion) None
acid Hydrophobic (R) (pK 2.5–5) Absorption (R) Ionic
a
G Alkene Hydrophobic Neutral Absorption Hydrophobic None
Chapter 2.10 (remove bold from drug name) van der Waals
D (four membered ring)
B
E
A
C G
F
Cefprozil
2. Based on the information in the structure evaluation grid, determine if cefprozil is an acidic, basic, or
amphoteric drug. Provide a brief explanation for your answer.
Answer
Cefprozil contains two acidic functional groups (phenol and carboxylic acid) and one basic functional
group (primary amine). Because this drug contains both acidic and basic functional groups, it is
considered amphoteric in nature.