Page 191 - Medicinal Chemistry Self Assessment
P. 191
180 Medicinal Chemistry Self Assessment
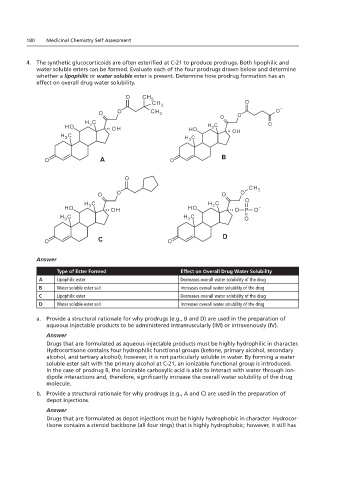
4. The synthetic glucocorticoids are often esterified at C-21 to produce prodrugs. Both lipophilic and
4. The synthetic n overall drug water solubility.
water soluble esters can be formed. Evaluate each of the four prodrugs drawn below and determine
whether a lipophilic or water soluble ester is present. Determine how prodrug formation has an
effect on overall drug water solubility.
A B
C D
Answer
Type of Ester Formed Effect on Overall Drug Water Solubility
A Lipophilic ester Decreases overall water solubility of the drug
B Water soluble ester salt Increases overall water solubility of the drug
C Lipophilic ester Decreases overall water solubility of the drug
D Water soluble ester salt Increases overall water solubility of the drug
a. Provide a structural rationale for why prodrugs (e.g., B and D) are used in the preparation of
aqueous injectable products to be administered intramuscularly (IM) or intravenously (IV).
Answer
Drugs that are formulated as aqueous injectable products must be highly hydrophilic in character.
Hydrocortisone contains four hydrophilic functional groups (ketone, primary alcohol, secondary
alcohol, and tertiary alcohol); however, it is not particularly soluble in water. By forming a water
soluble ester salt with the primary alcohol at C-21, an ionizable functional group is introduced.
In the case of prodrug B, the ionizable carboxylic acid is able to interact with water through ion–
dipole interactions and, therefore, significantly increase the overall water solubility of the drug
molecule.
b. Provide a structural rationale for why prodrugs (e.g., A and C) are used in the preparation of
depot injections.
Answer
Drugs that are formulated as depot injections must be highly hydrophobic in character. Hydrocor-
tisone contains a steroid backbone (all four rings) that is highly hydrophobic; however, it still has