Page 197 - Medicinal Chemistry Self Assessment
P. 197
4. T 3 is the biologically identified in the structure evaluation grid.
O
I
H
H
O
I
5. T 4 is biosynthesized from L-tyrosine within derived from L-tryosine.
I
N
H
2
OH
H O O I Tri-iodo-L-thyronine H O NH 2 OH I O OH
H NH
I O 2
OH I
Levothyroxine
L-Tyrosine 1.18 and 2.18 – remove bold from label
186 Medicinal Chemistry Self Assessment
Answer: I O
H O I
The biosynthesis s found in the amino acid tyrosine.
evident by the presence of only the phenol portion of the amino acid side chain. Box B is also derived OH
from L-tyrosine; however, the phenol has become an ether. The rest of the boxed atoms represent H
each of the atoms found in the amino acid tyrosine. O NH 2
I
A I I B O O A
H O H O I I
OH OH
NH H NH
I I O O 2 2 I O
I I H O I
OH
Levothyroxine
Levothyroxine
Levothyroxine H NH
I O 2
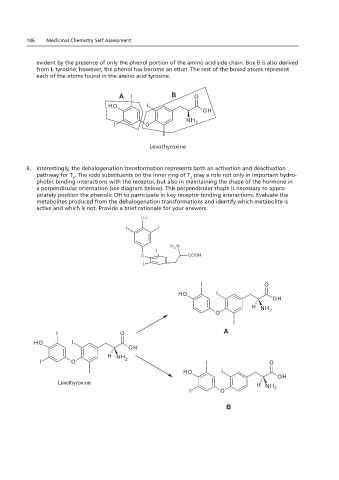
6. Interestingly, the dehalogenation transformation represents both an activation and deactivation B
pathway for T . The iodo substituents on the inner ring of T play a role not only in important hydro-
4
4
phobic binding interactions with the receptor, but also in maintaining the shape of the hormone in
a perpendicular orientation (see diagram below). This perpendicular shape is necessary to appro-
1.18 and 2.18 – structure was fixed (pay no attention to the colors)
priately position the phenolic OH to participate in key receptor binding interactions. Evaluate the
metabolites produced from the dehalogenation transformations and identify which metabolite is
active and which is not. Provide a brief rationale for your answers.
1.18 and 2.18 – remove bold from label
I O
1.19 and 2.19 – remove bold from label.
I
H
O
OH
H
A O NH 2
C
I
I O D A
H O I
OH
H NH B
I O 2 I O
I Lidocaine H O I
OH
Levothyroxine H NH
I O 2
B
1.18 and 2.18 – structure was fixed (pay no attention to the colors)
1.19 and 2.19 – remove bold from label.
A
C
D
B
Lidocaine