Page 205 - Medicinal Chemistry Self Assessment
P. 205
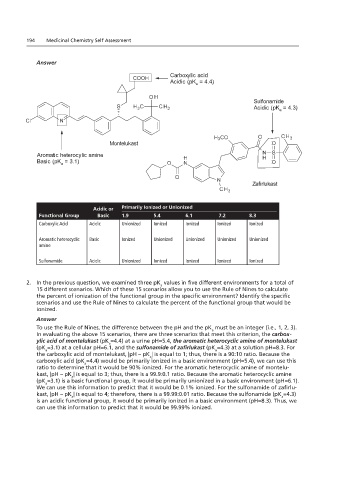
Montelukast
Zafirlukast
194 Medicinal Chemistry Self Assessment
1. The structure of a solution pH of 8.3.
Answer:
Answer
Carboxylic acid
Acidic (pK = 4.4)
a
Sulfonamide
Acidic (pK = 4.3)
a
Montelukast
Aromatic heterocylic amine
Basic (pK = 3.1)
a
Zafirlukast
Acidic or Primarily Ionized or Unionized
Functional Group Basic 1.9 5.4 6.1 7.2 8.3
Carboxylic Acid Acidic Unionized Ionized Ionized Ionized Ionized
2. In the previous question, we to calculate the percent of the functional group that would be ionized.
3. The sodium salt an explanation for this difference.
Aromatic heterocyclic Basic Ionized Unionized Unionized Unionized Unionized
amine
Sulfonamide Acidic Unionized Ionized Ionized Ionized Ionized
2. In the previous question, we examined three pK values in five different environments for a total of
a
15 different scenarios. Which of these 15 scenarios allow you to use the Rule of Nines to calculate
the percent of ionization of the functional group in the specific environment? Identify the specific
scenarios and use the Rule of Nines to calculate the percent of the functional group that would be
ionized.
Answer
To use the Rule of Nines, the difference between the pH and the pK must be an integer (i.e., 1, 2, 3).
a
In evaluating the above 15 scenarios, there are three scenarios that meet this criterion, the carbox-
ylic acid of montelukast (pK =4.4) at a urine pH=5.4, the aromatic heterocyclic amine of montelukast
a
(pK =3.1) at a cellular pH=6.1, and the sulfonamide of zafirlukast (pK =4.3) at a solution pH=8.3. For
a
a
the carboxylic acid of montelukast, |pH – pK | is equal to 1; thus, there is a 90:10 ratio. Because the
a
carboxylic acid (pK =4.4) would be primarily ionized in a basic environment (pH=5.4), we can use this
a
ratio to determine that it would be 90% ionized. For the aromatic heterocyclic amine of montelu-
kast, |pH – pK | is equal to 3; thus, there is a 99.9:0.1 ratio. Because the aromatic heterocyclic amine
a
(pK =3.1) is a basic functional group, it would be primarily unionized in a basic environment (pH=6.1).
a
We can use this information to predict that it would be 0.1% ionized. For the sulfonamide of zafirlu-
kast, |pH – pK | is equal to 4; therefore, there is a 99.99:0.01 ratio. Because the sulfonamide (pK =4.3)
a
a
is an acidic functional group, it would be primarily ionized in a basic environment (pH=8.3). Thus, we
can use this information to predict that it would be 99.99% ionized.