Page 208 - Medicinal Chemistry Self Assessment
P. 208
2.20 Montelukast and Zafirlukast 197
5. Calculated log P values of montelukast and zafirlukast lie in the range of 5.5 to 6.4 depending on the
computer program used to predict these values. Given this information, would these drug molecules
be predicted to be highly plasma protein bound or minimally plasma protein bound? Additionally,
would you expect these drug molecules to undergo extensive hepatic metabolism or be primarily
excreted unchanged?
Similarly, the structure of group that could participate in an ionic bond with the leukotriene.
Answer
Plasma proteins are used by the human body to transport endogenous molecules in the plasma from
Hydrogen bond
one cell to another. Given that the plasma is water soluble, these proteins are primarily required to
(as acceptor)
carry those endogenous molecules that have a high level of lipid solubility (e.g., estradiol, choles-
The four boxed areas could interact with a
terol, hydrocortisone). This is also true for exogenously administered drug molecules. Drug molecules
hydrophobic pocket on the leukotriene receptors.
that have a more lipid soluble character have a greater affinity for plasma proteins than do those
that have a more water soluble character. Thus, using the calculated log P values provided for monte-
lukast and zafirlukast, it would be expected that these two drug molecules would be highly plasma
protein bound.
The primary purpose of drug metabolism is to enhance the removal of the drug molecule from the
Ionic bond
human body. Drug molecules that already possess adequate water solubility, and thus can be readily
eliminated, generally undergo minimal or no metabolism, whereas drug molecules that are highly
lipid soluble often undergo extensive metabolic transformation. Thus, using the calculated log P
values provided for montelukast and zafirlukast, it would be expected that these two drug molecules
would undergo extensive hepatic metabolism. Zafirlukast
Hydrogen bond
(as acceptor)
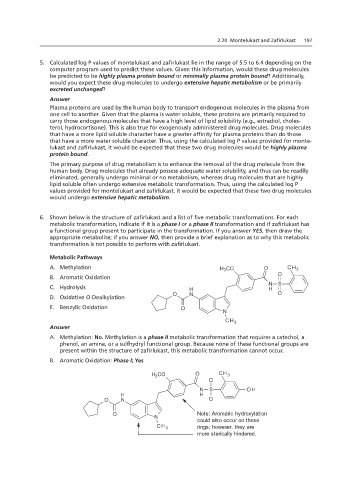
6. Shown below is the structure of zafirlukast and a list of five metabolic transformations. For each
metabolic transformation, indicate if it is a phase I or a phase II transformation and if zafirlukast has
5. Calculated log P values of montelukast or be primarily excreted unchanged?
a functional group present to participate in the transformation. If you answer YES, then draw the
appropriate metabolite; if you answer NO, then provide a brief explanation as to why this metabolic
transformation is not possible to perform with zafirlukast.
6. Shown below is the to perform with zafirlukast.
Metabolic Pathways
A. Methylation
B. Aromatic Oxidation
C. Hydrolysis
D. Oxidative O-Dealkylation
Replacement Structures for Batch 3
E. Benzylic Oxidation
Chapter 2.20
Answer
Please replace the structure for answer 6B (page 5) with the one shown below.
A. Methylation: No. Methylation is a phase II metabolic transformation that requires a catechol, a
phenol, an amine, or a sulfhydryl functional group. Because none of these functional groups are
Answer:
present within the structure of zafirlukast, this metabolic transformation cannot occur.
B. Aromatic Oxidation: Phase I; Yes
A. Methylation: No., this metabolic transformation cannot occur.
B. Aromatic Oxidation: Phase I; Yes
Note: Aromaric hydroxylation
could also occur on these
rings; however, they are
more sterically hindered.
Note: Aromaric hydroxylation
could also occur on this
ring; however, it is more
Please replace the structure for answer 6C (page 6) with the one shown below.
sterically hindered.
H CO O CH 3
3
O
OH
N S
H H
H O N O
O N Note: It is also possible to
hydrolyze the sulfonamide.
CH 3