Page 157 - Basic _ Clinical Pharmacology ( PDFDrive )
P. 157
CHAPTER 9 Adrenoceptor Agonists & Sympathomimetic Drugs 143
A B
Postganglionic
sympathetic
nerve ending
NET
NET
NE VMAT Amphetamine
VMAT
NE
NET
NET
Reversed
transport
NE
NE
Effector cell Effector cell
C
NET
Cocaine VMAT
NE
NET Blocked
transport
NE
Effector cell
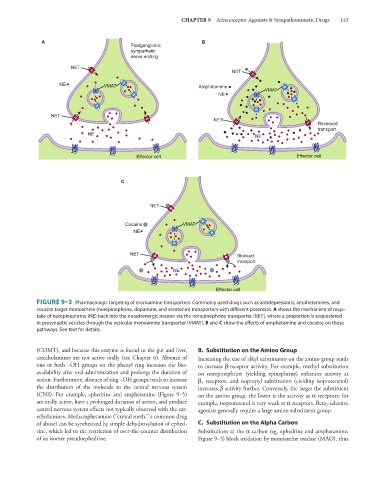
FIGURE 9–3 Pharmacologic targeting of monoamine transporters. Commonly used drugs such as antidepressants, amphetamines, and
cocaine target monoamine (norepinephrine, dopamine, and serotonin) transporters with different potencies. A shows the mechanism of reup-
take of norepinephrine (NE) back into the noradrenergic neuron via the norepinephrine transporter (NET), where a proportion is sequestered
in presynaptic vesicles through the vesicular monoamine transporter (VMAT). B and C show the effects of amphetamine and cocaine on these
pathways. See text for details.
(COMT), and because this enzyme is found in the gut and liver, B. Substitution on the Amino Group
catecholamines are not active orally (see Chapter 6). Absence of Increasing the size of alkyl substituents on the amino group tends
one or both –OH groups on the phenyl ring increases the bio- to increase β-receptor activity. For example, methyl substitution
availability after oral administration and prolongs the duration of on norepinephrine (yielding epinephrine) enhances activity at
action. Furthermore, absence of ring –OH groups tends to increase β receptors, and isopropyl substitution (yielding isoproterenol)
2
the distribution of the molecule to the central nervous system increases β activity further. Conversely, the larger the substituent
(CNS). For example, ephedrine and amphetamine (Figure 9–5) on the amino group, the lower is the activity at α receptors; for
are orally active, have a prolonged duration of action, and produce example, isoproterenol is very weak at α receptors. Beta -selective
2
central nervous system effects not typically observed with the cat- agonists generally require a large amino substituent group.
echolamines. Methamphetamine (“crystal meth,” a common drug
of abuse) can be synthesized by simple dehydroxylation of ephed- C. Substitution on the Alpha Carbon
rine, which led to the restriction of over-the-counter distribution Substitutions at the α carbon (eg, ephedrine and amphetamine;
of its isomer pseudoephedrine. Figure 9–5) block oxidation by monoamine oxidase (MAO), thus