Page 16 - Ilmu Tanah
P. 16
The Chemistry and Fertility of Soils under Tropical Weeds 3
low pH and Fe(OH) 2 at high pH (Salam, 2017) as shown in Fig. 1.1. This shows that
the existence of Fe species depends on and is governed by the two major soil
variables i.e. soil pH and soil E. Alkalization/acidification and draining/flooding are
the important techniques to manage the Fe species and many other heavy metal
-
elements subjected to precipitation/dissolution reactions by OH ions and
oxidation/reduction reactions.
1)
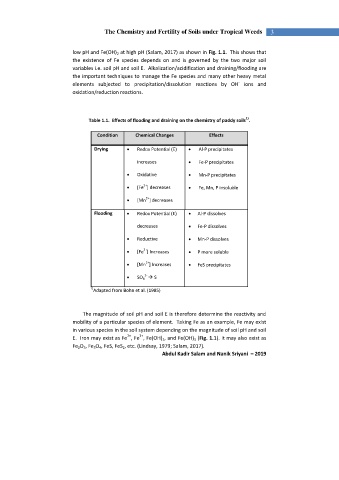
Table 1.1. Effects of flooding and draining on the chemistry of paddy soils .
Condition Chemical Changes Effects
Drying Redox Potential (E) Al-P precipitates
increases Fe-P precipitates
Oxidative Mn-P precipitates
2+
[Fe ] decreases Fe, Mn, P insoluble
2+
[Mn ] decreases
Flooding Redox Potential (E) Al-P dissolves
decreases Fe-P dissolves
Reductive Mn-P dissolves
2+
[Fe ] Increases P more soluble
2+
[Mn ] Increases FeS precipitates
2-
SO 4 S
1)
Adapted from Bohn et al. (1985)
The magnitude of soil pH and soil E is therefore determine the reactivity and
mobility of a particular species of element. Taking Fe as an example, Fe may exist
in various species in the soil system depending on the magnitude of soil pH and soil
2+
3+
E. Iron may exist as Fe , Fe , Fe(OH) 2 , and Fe(OH) 3 (Fig. 1.1). It may also exist as
Fe 2 O 3 , Fe 3 O 4 , FeS, FeS 2 , etc. (Lindsay, 1979; Salam, 2017).
Abdul Kadir Salam and Nanik Sriyani – 2019