Page 17 - Ilmu Tanah
P. 17
4 The Chemistry and Fertility of Soils under Tropical Weeds
E (V)
1.23
Oxidative
Fe 3+
0.77
Fe(OH)3
0.27 Fe 2+
0.00 Fe(OH)2
Reductive
0 0.87 7 7.15 pH
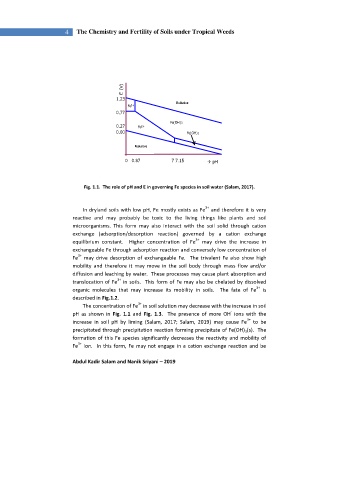
Fig. 1.1. The role of pH and E in governing Fe species in soil water (Salam, 2017).
3+
In dryland soils with low pH, Fe mostly exists as Fe and therefore it is very
reactive and may probably be toxic to the living things like plants and soil
microorganisms. This form may also interact with the soil solid through cation
exchange (adsorption/desorption reaction) governed by a cation exchange
3+
equilibrium constant. Higher concentration of Fe may drive the increase in
exchangeable Fe through adsorption reaction and conversely low concentration of
3+
Fe may drive desorption of exchangeable Fe. The trivalent Fe also show high
mobility and therefore it may move in the soil body through mass flow and/or
diffusion and leaching by water. These processes may cause plant absorption and
3+
translocation of Fe in soils. This form of Fe may also be chelated by dissolved
3+
organic molecules that may increase its mobility in soils. The fate of Fe is
described in Fig.1.2.
3+
The concentration of Fe in soil solution may decrease with the increase in soil
-
pH as shown in Fig. 1.1 and Fig. 1.3. The presence of more OH ions with the
3+
increase in soil pH by liming (Salam, 2017; Salam, 2019) may cause Fe to be
precipitated through precipitation reaction forming precipitate of Fe(OH) 3 (s). The
formation of this Fe species significantly decreases the reactivity and mobility of
3+
Fe ion. In this form, Fe may not engage in a cation exchange reaction and be
Abdul Kadir Salam and Nanik Sriyani – 2019