Page 19 - Ilmu Tanah
P. 19
6 The Chemistry and Fertility of Soils under Tropical Weeds
0.012
0.01
[Fe 2+ or Fe 3+ ] 0.006
0.008
0.004
0.002
0
4 5 6 7
pH
2+ 3+
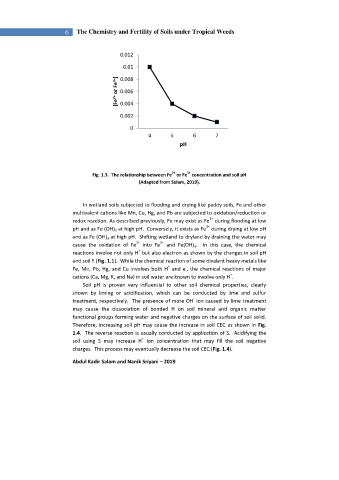
Fig. 1.3. The relationship between Fe or Fe concentration and soil pH
(Adapted from Salam, 2019).
In wetland soils subjected to flooding and drying like paddy soils, Fe and other
multivalent cations like Mn, Cu, Hg, and Pb are subjected to oxidation/reduction or
2+
redox reaction. As described previously, Fe may exist as Fe during flooding at low
3+
pH and as Fe (OH) 2 at high pH. Conversely, it exists as Fe during drying at low pH
and as Fe (OH) 3 at high pH. Shifting wetland to dryland by draining the water may
2+
3+
cause the oxidation of Fe into Fe and Fe(OH) 3 . In this case, the chemical
+
reactions involve not only H but also electron as shown by the changes in soil pH
and soil E (Fig. 1.1). While the chemical reaction of some divalent heavy metals like
-
+
Fe, Mn, Pb, Hg, and Cu involves both H and e , the chemical reactions of major
+
cations (Ca, Mg, K, and Na) in soil water are known to involve only H .
Soil pH is proven very influential to other soil chemical properties, clearly
shown by liming or acidification, which can be conducted by lime and sulfur
-
treatment, respectively. The presence of more OH ion caused by lime treatment
may cause the dissociation of bonded H on soil mineral and organic matter
functional groups forming water and negative charges on the surface of soil solid.
Therefore, increasing soil pH may cause the increase in soil CEC as shown in Fig.
1.4. The reverse reaction is usually conducted by application of S. Acidifying the
+
soil using S may increase H ion concentration that may fill the soil negative
charges. This process may eventually decrease the soil CEC (Fig. 1.4).
Abdul Kadir Salam and Nanik Sriyani – 2019