Page 41 - Ilmu Tanah Book
P. 41
28 The Chemistry and Fertility of Soils under Tropical Weeds
cause the absence of the related nutrient cations and nutrient element
deficiencies.
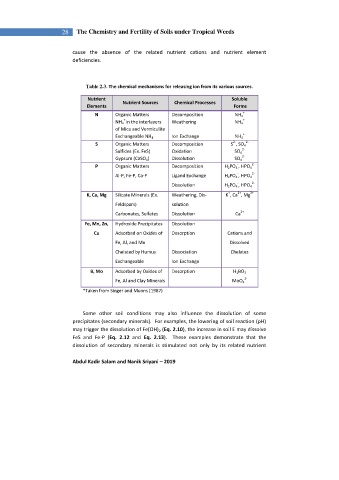
Table 2.3. The chemical mechanisms for releasing ion from its various sources.
Nutrient Soluble
Nutrient Sources Chemical Processes
Elements Forms
N Organic Matters Decomposition NH 4 +
+ +
NH 4 in the interlayers Weathering NH 4
of Mica and Vermiculite
+
Exchangeable NH 4 Ion Exchange NH 4
2- 2-
S Organic Matters Decomposition S , SO 4
Sulfides (Ex. FeS) Oxidation SO 4 2-
2-
Gypsum (CaSO 4 ) Dissolution SO 4
- 2-
P Organic Matters Decomposition H 2 PO 4 , HPO 4
-
Al-P, Fe-P, Ca-P Ligand Exchange H 2 PO 4 , HPO 4 2-
- 2-
Dissolution H 2 PO 4 , HPO 4
+
2+
2+
K, Ca, Mg Silicate Minerals (Ex. Weathering, Dis- K , Ca , Mg
Feldspars) solution
2+
Carbonates, Sulfates Dissolution Ca
Fe, Mn, Zn, Hydroxide Precipitates Dissolution
Cu Adsorbed on Oxides of Desorption Cations and
Fe, Al, and Mn Dissolved
Chelated by Humus Dissociation Chelates
Exchangeable Ion Exchange
B, Mo Adsorbed by Oxides of Desorption H 3 BO 3
2-
Fe, Al and Clay Minerals MoO 4
*Taken from Singer and Munns (1987)
Some other soil conditions may also influence the dissolution of some
precipitates (secondary minerals). For examples, the lowering of soil reaction (pH)
may trigger the dissolution of Fe(OH) 2 (Eq. 2.10), the increase in soil E may dissolve
FeS and Fe-P (Eq. 2.12 and Eq. 2.13). These examples demonstrate that the
dissolution of secondary minerals is stimulated not only by its related nutrient
Abdul Kadir Salam and Nanik Sriyani – 2019