Page 210 - Fiber Optic Communications Fund
P. 210
Optical Receivers 191
Photon energy (eV)
3 2 1 0.7
10 6
Ge
10 5 In 0.7 Ga 0.3 As 0.64 P 0.36
Absorption coefficient (cm −1 ) 10 4 3 Si GaAs
In 0.53 Ga 0.47 As
InP
10
10 2 a–Si:H
10
0.2 0.6 1.0 1.4 1.8
Wavelength (μm)
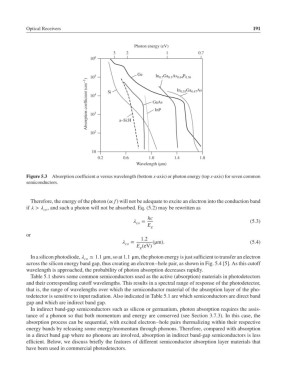
Figure 5.3 Absorption coefficient versus wavelength (bottom x-axis) or photon energy (top x-axis) for seven common
semiconductors.
Therefore, the energy of the photon (∝ f) will not be adequate to excite an electron into the conduction band
if > , and such a photon will not be absorbed. Eq. (5.2) may be rewritten as
co
hc
co = (5.3)
E g
or
1.2
co = (μm). (5.4)
E (eV)
g
In a silicon photodiode, ≃ 1.1 μm, so at 1.1 μm, the photon energy is just sufficient to transfer an electron
co
across the silicon energy band gap, thus creating an electron–hole pair, as shown in Fig. 5.4 [5]. As this cutoff
wavelength is approached, the probability of photon absorption decreases rapidly.
Table 5.1 shows some common semiconductors used as the active (absorption) materials in photodetectors
and their corresponding cutoff wavelengths. This results in a spectral range of response of the photodetector,
that is, the range of wavelengths over which the semiconductor material of the absorption layer of the pho-
todetector is sensitive to input radiation. Also indicated in Table 5.1 are which semiconductors are direct band
gap and which are indirect band gap.
In indirect band-gap semiconductors such as silicon or germanium, photon absorption requires the assis-
tance of a phonon so that both momentum and energy are conserved (see Section 3.7.3). In this case, the
absorption process can be sequential, with excited electron–hole pairs thermalizing within their respective
energy bands by releasing some energy/momentum through phonons. Therefore, compared with absorption
in a direct band gap where no phonons are involved, absorption in indirect band-gap semiconductors is less
efficient. Below, we discuss briefly the features of different semiconductor absorption layer materials that
have been used in commercial photodetectors.