Page 48 - E-Modul Asam Basa Berbasis Guided Discovery Learning
P. 48
E E-MODUL ASAM & BASA
u
i
G
d
d
e
s
e
r
Berbasis Guided Discovery Learning
B
s
i
b
a
e
a
L
r
n
g
n
i
s
c
D
i
o
r
y
v
e
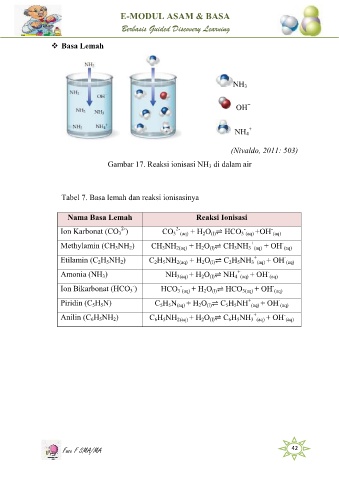
Basa Lemah
NH
3 3
OH −
O
H
H
NH 4 +
N
o
(Nivaldo, 2011: 503)
)
0
:
2
3
,
5
0
1
i
N
v
1
l
a
(
d
Gambar 17. Reaksi ionisasi NH di dalam air
G a m b a r 1 7 . R e a k s i i o n i s a s i N H 3 d i d a l a m a i r
i
d
r
k
s
n
a
.
7
e
a
a
Tabel 7. Basa lemah dan reaksi ionisasinya
e
l
m
h
a
a
s
B
m
Nama Basa Lemah R e a k s i I o n i s a s i
Reaksi Ionisasi
a
N
h
m
e
a
B
L
a
a
s
a
2 2-
- -
2-
Ion Karbonat (CO ) CO 3 (aq) + H O ⇌ HCO 3 (aq) +OH - (aq)
(l)
2
3
+
Methylamin (CH NH ) CH NH 2(aq) + H O ⇌ CH NH 3 (aq) + OH - (aq)
N
N
H
H
2
3
3
2
3
(l)
+
H
Etilamin (C H NH ) C H NH 2(aq) + H O ⇌ C H NH 3 (aq) + OH - (aq)
N
2
2 2
5
(l)
2
2
5
5
2
+
Amonia (NH ) NH 3(aq) + H O ⇌ NH 4 (aq) + OH - (aq)
3
a
2
)
q
(
(l)
-
-
a
o
B
n
i
k
o
r
Ion Bikarbonat (HCO ) HCO 3 (aq) + H O ⇌ HCO 3 3(aq) + OH - (aq)
O
b
n
C
I
H
(
a
t
a
(l)
)
3
2
q
(
Piridin (C H N) C H N (aq) + H O ⇌ C H NH + (aq) + OH - (aq)
N
H
5
5
5
5
2
(l)
5
5
+
N
Anilin (C H NH ) C H NH 2(aq) + H O ⇌ C H NH 3 (aq) + OH - (aq)
H
2
6
6
5
5
6
5
2
(l)
Fase F SMA/MA 42