Page 157 - 00. Complete Version - Progress Report IPEN 2014-2016
P. 157
Nuclear Science and Technology | Progress Report 157
Rietveld analyses of neutron and ple resulted Ba0.45Sr0.55Co0.80Fe0.20O2.427
X-ray diffraction patterns (δ = 0.573).
The Neutron Diffraction Laboratory is also
involved in studies that employ the Rietveld
quantitative phase analysis using neutron and
X-ray diffraction patterns. The studies present-
ed below were all made in cooperation with
other groups. The X-ray diffraction patterns
were measured at different laboratories, in-
side and outside IPEN, and the neutron dif-
fraction ones in the high-resolution diffrac-
tometer Aurora.
Oxygen stoichiometry in BSCF
In a co-operative study between the Science
and Technology of Materials Centre and the
Neutron Diffraction Laboratory, the compound
Ba0.50Sr0.50Co0.80Fe0.20O3-δ (BSCF) has
been studied. BSCF is a candidate for air elec-
trodes in Intermediate Temperature Solid Ox-
ide Fuel Cells (ITSOFC). This work deals with
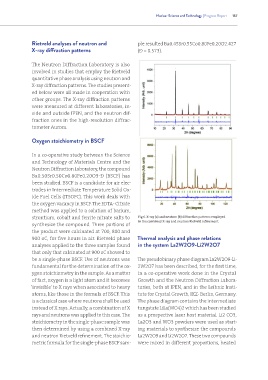
the oxygen vacancy in BSCF. The EDTA- Citrate
method was applied to a solution of barium,
strontium, cobalt and ferrite nitrate salts to Fig 6. X-ray (a) and neutron (b) diffraction patterns employed
in the combined X-ray and neutron Rietveld refinement.
synthesize the compound. Three portions of
the product were calcinated at 700, 800 and
900 oC, for five hours in air. Rietveld phase Thermal analysis and phase relations
analyses applied to the three samples found in the system La2W2O9-Li2W2O7
that only that calcinated at 900 oC showed to
be a single-phase BSCF. Use of neutrons was The pseudobinary phase diagram La2W2O9-Li-
fundamental for the determination of the ox- 2W2O7 has been described, for the first time,
ygen stoichiometry in the sample. As a matter in a co-operative work done in the Crystal
of fact, oxygen is a light atom and it becomes Growth and the Neutron Diffraction Labora-
‘invisible’ to X rays when associated to heavy tories, both at IPEN, and in the Leibniz Insti-
atoms, like those in the formula of BSCF. This tute for Crystal Growth, IKZ- Berlin, Germany.
is a classical case where neutrons shall be used The phase diagram contains the intermediate
instead of X rays. Actually, a combination of X tungstate LiLa(WO4)2 which has been studied
rays and neutrons was applied to this case. The as a prospective laser host material. Li2 CO3,
stoichiometry in the single-phase sample was La2O3 and WO3 powders were used as start-
then determined by using a combined X-ray ing materials to synthesize the compounds
and neutron Rietveld refinement. The stoichio- La2W2O9 and Li2W2O7. These two compounds
metric formula for the single-phase BSCF sam- were mixed in different proportions, heated