Page 158 - 00. Complete Version - Progress Report IPEN 2014-2016
P. 158
158 Nuclear Science and Technology | Progress Report
to the melting temperatures and cooled with
a constant rate to produce annealed samples
for the study. X-ray diffraction data for Riet-
veld analyses were collected at room tempera-
ture. Four different phases were identified in
the system xLi2W2O7 - (1-x) La2W2O9 for 0≤
x ≤1, namely LiLa(WO4)2, La2W2O9, Li2W2O7
and Li2WO4. In the interval 0< x <1, phase
LiLa(WO4)2 is formed for all values of x. One,
two or even three of the other phases coexist
with LiLa(WO4)2 in shorter intermediate in-
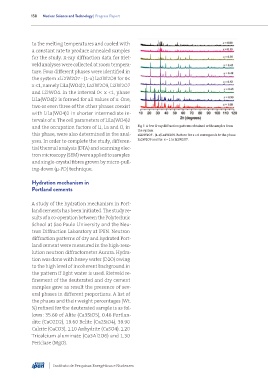
tervals of x. The cell parameters of LiLa(WO4)2
and the occupation factors of Li, La and O, in Fig 7. A few X-ray diffraction patterns obtained with samples from
the system
this phase, were also determined in the anal- xLi2W2O7 - (1-x)La2W2O9. Pattern for x = 0 corresponds to the phase
yses. In order to complete the study, differen- La2W2O9 and for x = 1 to Li2W2O7.
tial thermal analysis (DTA) and scanning elec-
tron microscopy (SEM) were applied to samples
and single-crystal fibres grown by micro-pull-
ing-down (μ-PD) technique.
Hydration mechanism in
Portland cements
A study of the hydration mechanism in Port-
land cements has been initiated. The study re-
sults of a co-operation between the Polytechnic
School at Sao Paulo University and the Neu-
tron Diffraction Laboratory at IPEN. Neutron
diffraction patterns of dry and hydrated Port-
land cement were measured in the high-reso-
lution neutron diffractometer Aurora. Hydra-
tion was done with heavy water (D2O) owing
to the high level of incoherent background in
the pattern if light water is used. Rietveld re-
finement of the deuterated and dry cement
samples gave as result the presence of sev-
eral phases in different proportions. A list of
the phases and their weight percentages (Wt.
%) refined for the deuterated sample is as fol-
lows: 35.60 of Alite (Ca3SiO5), 0.46 Portlan-
dite (CaO2D2), 19.60 Belite (Ca2SiO4), 39.90
Calcite (CaCO3), 1.10 Anhydrite (CaSO4), 1.20
Tricalcium aluminate (Ca3Al2O6) and 1.30
Periclase (MgO).
Instituto de Pesquisas Energéticas e Nucleares