Page 302 - 00. Complete Version - Progress Report IPEN 2014-2016
P. 302
302 Materials and Nanotechnology | Progress Report
Materials and Technologies (UFSCar). We verified that non-stoichiometric
for a Self-Sustained TiFe can be prepared by appropriated change
Environment of the relative quantities of titanium and iron
powders for milling. Non-stoichiometric TiFe
could absorb and desorb hydrogen at lower
Materials for Hydrogen Storage pressures and higher rates compared to the
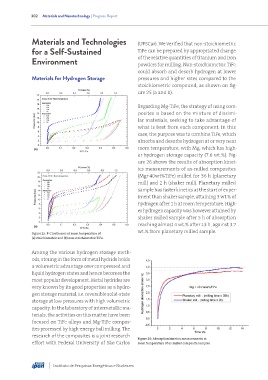
stoichiometric compound, as shown on fig-
ure 25 (a and b).
Regarding Mg-TiFe, the strategy of using com-
posites is based on the mixture of dissimi-
lar materials, seeking to take advantage of
what is best from each component. In this
case, the purpose was to combine TiFe, which
absorbs and desorbs hydrogen at or very near
room temperature, with Mg, which has high-
er hydrogen storage capacity (7.6 wt.%). Fig-
ure 26 shows the results of absorption kinet-
ics measurements of as-milled composites
(Mg+40wt%TiFe) milled for 36 h (planetary
mill) and 2 h (shaker mill). Planetary milled
sample has faster kinetics at the start of exper-
iment than shaker sample, attaining 3 wt.% of
hydrogen after 1 h at room temperature. High-
er hydrogen capacity was however attained by
shaker milled sample after 5 h of absorption
reaching almost 4 wt.% after 13 h, against 3.7
wt.% from planetary milled sample.
Figure 25: P-C isotherms at room temperature of
(a) stoichiometric and (b) non-stoichiometric TiFe.
Among the various hydrogen storage meth-
ods, storing in the form of metal hydride holds
a volumetric advantage over compressed and
liquid hydrogen states and hence becomes the
most popular development. Metal hydrides are
very known by its good properties as a hydro-
gen storage material, i.e. reversible solid-state
storage at low pressures with high volumetric
capacity. In the laboratory of intermetallic ma-
terials, the activities on this matter have been
focused on TiFe alloys and Mg-TiFe compos-
ites processed by high-energy ball milling. The
research of the composites is a joint research
Figure 26: Absorption kinetics measurements at
effort with Federal University of São Carlos room temperature of as-milled composite samples
Instituto de Pesquisas Energéticas e Nucleares