Page 17 - Laboratory manual for students FAR222 2019 20
P. 17
FAR 222 Dosage Form II Laboratory Manual
5 THE PRODUCTION OF STERILE PRODUCTS
Sterile products should be manufactured with special care and attention to detail, with the
object of eliminating microbial and particulate contamination. Much depends on the skill,
training and attitudes of the personnel involved. Even more than with other types of medicinal
product, it is not sufficient that the finished product passes the specified tests, and products
may be classified into two categories according to their manner of production, namely:
those which are sterilised in their final containers (terminally sterilised). This is the
method of choice whenever possible
those which are prepared under aseptic conditions from previously sterilised materials
(non-terminally sterilised)
Terminally sterilised products should be manufactured in a ‘clean area’.
This is a room with defined environmental control of particulate and microbial contamination
constructed and used so as to reduce the introduction, generation and retention of
contaminants within the area.
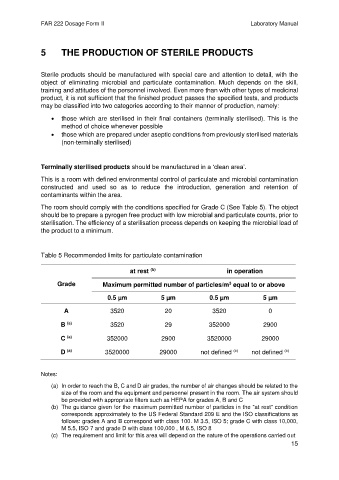
The room should comply with the conditions specified for Grade C (See Table 5). The object
should be to prepare a pyrogen free product with low microbial and particulate counts, prior to
sterilisation. The efficiency of a sterilisation process depends on keeping the microbial load of
the product to a minimum.
Table 5 Recommended limits for particulate contamination
(b)
at rest in operation
Grade Maximum permitted number of particles/m equal to or above
3
0.5 µm 5 µm 0.5 µm 5 µm
A 3520 20 3520 0
(a)
B 3520 29 352000 2900
(a)
C 352000 2900 3520000 29000
(c)
(a)
(c)
D 3520000 29000 not defined not defined
Notes:
(a) In order to reach the B, C and D air grades, the number of air changes should be related to the
size of the room and the equipment and personnel present in the room. The air system should
be provided with appropriate filters such as HEPA for grades A, B and C
(b) The guidance given for the maximum permitted number of particles in the "at rest" condition
corresponds approximately to the US Federal Standard 209 E and the ISO classifications as
follows: grades A and B correspond with class 100. M 3.5, ISO 5; grade C with class 10,000,
M 5.5, ISO 7 and grade D with class 100,000 , M 6.5, ISO 8
(c) The requirement and limit for this area will depend on the nature of the operations carried out
15