Page 18 - Laboratory manual for students FAR222 2019 20
P. 18
FAR 222 Dosage Form II Laboratory Manual
Non-terminally sterilised products i.e. those prepared under aseptic conditions from
previously sterilised materials, can be processed in clean areas until they have been sterilised
but must subsequently be filled into the final sterile containers in ‘aseptic areas’. An ‘aseptic
area’ is a room, suite of rooms or, more usually, a special area within a clean area designed,
constructed, serviced and used with the intention of preventing microbial contamination of the
product. Such a room should comply, in the unmanned state, with the conditions specified for
Grade B (see Table 5). With people present and work in progress Grade A conditions should
be maintained under contained work stations where products are exposed and aseptic
manipulations carried out.
STERILISATION METHODS
Sterilisation is the process used to free the product from microorganisms. The purpose is to
provide a product, which may be described as sterile. Whatever their nature and resistance to
the sterilising process the fewer microorganisms on the product to be sterilised, the higher is
the probability of sterility after processing. All sterilisation processes have a finite capability for
destroying microorganisms. The effectiveness of the established process has been defined
using specified test organisms.
Commonly used sterilisation methods in the practical classes are:
i. Autoclaving (steam sterilisation)
Products that can withstand steam under pressure should be sterilised by this method. This
method should be used whenever possible for aqueous preparation and surgical dressing.
The sterility is achieved by use of dry saturated steam under pressure in a suitable designed
chamber. Various temperatures and periods of treatment are recommended in official
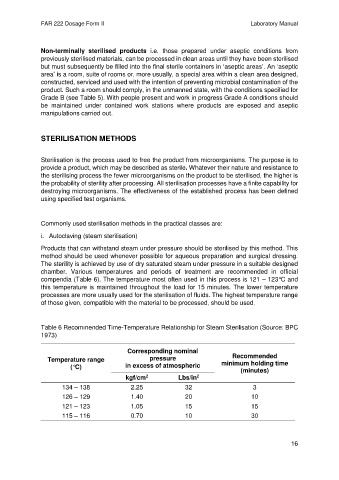
compendia (Table 6). The temperature most often used in this process is 121 – 123°C and
this temperature is maintained throughout the load for 15 minutes. The lower temperature
processes are more usually used for the sterilisation of fluids. The highest temperature range
of those given, compatible with the material to be processed, should be used.
Table 6 Recommended Time-Temperature Relationship for Steam Sterilisation (Source: BPC
1973)
Corresponding nominal
Recommended
Temperature range pressure minimum holding time
(°C) in excess of atmospheric
(minutes)
kgf/cm Lbs/in
2
2
134 – 138 2.25 32 3
126 – 129 1.40 20 10
121 – 123 1.05 15 15
115 – 116 0.70 10 30
16