Page 19 - Laboratory manual for students FAR222 2019 20
P. 19
FAR 222 Dosage Form II Laboratory Manual
ii. Dry heat sterilisation
Sterilisation by dry heat is usually carried out in a hot air oven. This method of heating is
utilised to sterilise products such as oils, powders and certain impregnated dressing. Cycles
recommended in the BP 1988 are shown in Table 7.
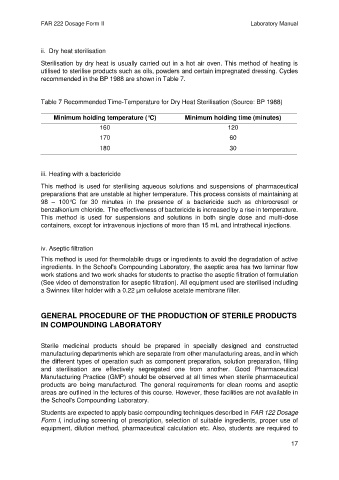
Table 7 Recommended Time-Temperature for Dry Heat Sterilisation (Source: BP 1988)
Minimum holding temperature (°C) Minimum holding time (minutes)
160 120
170 60
180 30
iii. Heating with a bactericide
This method is used for sterilising aqueous solutions and suspensions of pharmaceutical
preparations that are unstable at higher temperature. This process consists of maintaining at
98 – 100°C for 30 minutes in the presence of a bactericide such as chlorocresol or
benzalkonium chloride. The effectiveness of bactericide is increased by a rise in temperature.
This method is used for suspensions and solutions in both single dose and multi-dose
containers, except for intravenous injections of more than 15 mL and intrathecal injections.
iv. Aseptic filtration
This method is used for thermolabile drugs or ingredients to avoid the degradation of active
ingredients. In the School's Compounding Laboratory, the aseptic area has two laminar flow
work stations and two work shacks for students to practise the aseptic filtration of formulation
(See video of demonstration for aseptic filtration). All equipment used are sterilised including
a Swinnex filter holder with a 0.22 µm cellulose acetate membrane filter.
GENERAL PROCEDURE OF THE PRODUCTION OF STERILE PRODUCTS
IN COMPOUNDING LABORATORY
Sterile medicinal products should be prepared in specially designed and constructed
manufacturing departments which are separate from other manufacturing areas, and in which
the different types of operation such as component preparation, solution preparation, filling
and sterilisation are effectively segregated one from another. Good Pharmaceutical
Manufacturing Practice (GMP) should be observed at all times when sterile pharmaceutical
products are being manufactured. The general requirements for clean rooms and aseptic
areas are outlined in the lectures of this course. However, these facilities are not available in
the School's Compounding Laboratory.
Students are expected to apply basic compounding techniques described in FAR 122 Dosage
Form I, including screening of prescription, selection of suitable ingredients, proper use of
equipment, dilution method, pharmaceutical calculation etc. Also, students are required to
17