Page 65 - Cardiac Electrophysiology | A Modeling and Imaging Approach
P. 65
P. 65
of CaT when I phosphorylation is prevented results from
NaK
increased steady-state intracellular Na levels that reduce Ca
+
2+
extrusion via Na -Ca exchange.
2+
+
2.12. Calcium Cycling in Ventricular Myocytes:
Macroscopic Consequences of Microscopic
Dyadic Function 162
As was described in the previous section, in the
context of ßARS, the subcellular microscopic architecture of
the myocyte is an important determinant of its function. This
property is also of utmost importance for calcium cycling in
the cell. In cardiac ventricular myocytes, Ca release occurs
at local domains along T-tubules where L-type Ca channels
(LCCs) and RyR2s interact via Ca in dyadic spaces. In the
dyadic space, ~ 50 – 200 RyR2 in the terminal cisternae of the
SR, called junctional SR (JSR), closely appose 5 to 15 LCCs . Ca
163
release from the SR can occur in two ways: During excitation
– contraction coupling (ECC), LCCs open in response to the AP
and carry an influx of Ca into the dyadic space. This Ca influx
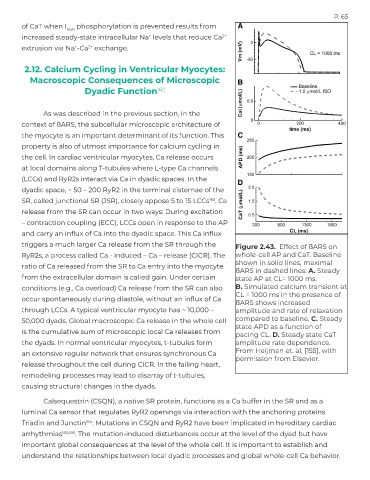
triggers a much larger Ca release from the SR through the Figure 2.43. Effect of ßARS on
RyR2s, a process called Ca - induced – Ca – release (CICR). The whole-cell AP and CaT. Baseline
shown in solid lines, maximal
ratio of Ca released from the SR to Ca entry into the myocyte
ßARS in dashed lines. A. Steady
from the extracellular domain is called gain. Under certain state AP at CL= 1000 ms.
conditions (e.g., Ca overload) Ca release from the SR can also B. Simulated calcium transient at
CL = 1000 ms in the presence of
occur spontaneously during diastole, without an influx of Ca
ßARS shows increased
through LCCs. A typical ventricular myocyte has ~ 10,000 – amplitude and rate of relaxation
50,000 dyads. Global macroscopic Ca release in the whole cell compared to baseline. C. Steady
state APD as a function of
is the cumulative sum of microscopic local Ca releases from pacing CL. D. Steady state CaT
the dyads. In normal ventricular myocytes, t-tubules form amplitude rate dependence.
an extensive regular network that ensures synchronous Ca From Heijman et. al. [155], with
permission from Elsevier.
release throughout the cell during CICR. In the failing heart,
remodeling processes may lead to disarray of t-tubules,
causing structural changes in the dyads.
Calsequestrin (CSQN), a native SR protein, functions as a Ca buffer in the SR and as a
luminal Ca sensor that regulates RyR2 openings via interaction with the anchoring proteins
Triadin and Junctin . Mutations in CSQN and RyR2 have been implicated in hereditary cardiac
164
arrhythmias 165,166 . The mutation-induced disturbances occur at the level of the dyad but have
important global consequences at the level of the whole cell. It is important to establish and
understand the relationships between local dyadic processes and global whole-cell Ca behavior.