Page 63 - Cardiac Electrophysiology | A Modeling and Imaging Approach
P. 63
P. 63
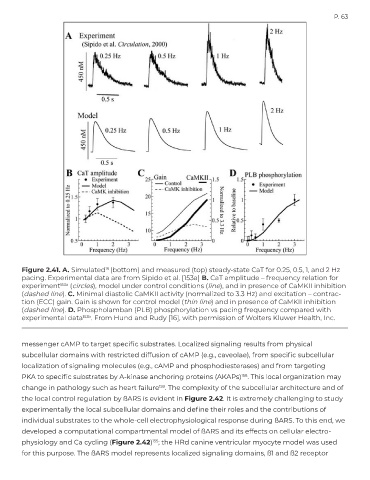
Figure 2.41. A. Simulated (bottom) and measured (top) steady-state CaT for 0.25, 0.5, 1, and 2 Hz
16
pacing. Experimental data are from Sipido et al. [153a] B. CaT amplitude – frequency relation for
experiment 153a (circles), model under control conditions (line), and in presence of CaMKII inhibition
(dashed line). C. Minimal diastolic CaMKII activity (normalized to 3.3 Hz) and excitation – contrac-
tion (ECC) gain. Gain is shown for control model (thin line) and in presence of CaMKII inhibition
(dashed line). D. Phospholamban (PLB) phosphorylation vs pacing frequency compared with
experimental data 153b . From Hund and Rudy [16], with permission of Wolters Kluwer Health, Inc.
messenger cAMP to target specific substrates. Localized signaling results from physical
subcellular domains with restricted diffusion of cAMP (e.g., caveolae), from specific subcellular
localization of signaling molecules (e.g., cAMP and phosphodiesterases) and from targeting
PKA to specific substrates by A-kinase anchoring proteins (AKAPs) . This local organization may
158
change in pathology such as heart failure . The complexity of the subcellular architecture and of
159
the local control regulation by ßARS is evident in Figure 2.42. It is extremely challenging to study
experimentally the local subcellular domains and define their roles and the contributions of
individual substrates to the whole-cell electrophysiological response during ßARS. To this end, we
developed a computational compartmental model of ßARS and its effects on cellular electro-
physiology and Ca cycling (Figure 2.42) ; the HRd canine ventricular myocyte model was used
155
for this purpose. The ßARS model represents localized signaling domains, ß1 and ß2 receptor