Page 1125 - Basic _ Clinical Pharmacology ( PDFDrive )
P. 1125
CHAPTER 62 Drugs Used in the Treatment of Gastrointestinal Diseases 1111
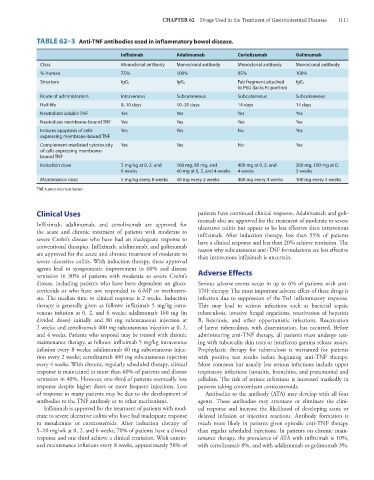
TABLE 62–3 Anti-TNF antibodies used in inflammatory bowel disease.
Infliximab Adalimumab Certolizumab Golimumab
Class Monoclonal antibody Monoclonal antibody Monoclonal antibody Monoclonal antibody
% Human 75% 100% 95% 100%
Structure IgG 1 IgG 1 Fab fragment attached IgG 1
to PEG (lacks Fc portion)
Route of administration Intravenous Subcutaneous Subcutaneous Subcutaneous
Half-life 8–10 days 10–20 days 14 days 14 days
Neutralizes soluble TNF Yes Yes Yes Yes
Neutralizes membrane-bound TNF Yes Yes Yes Yes
Induces apoptosis of cells Yes Yes No Yes
expressing membrane-bound TNF
Complement-mediated cytotoxicity Yes Yes No Yes
of cells expressing membrane-
bound TNF
Induction dose 5 mg/kg at 0, 2, and 160 mg, 80 mg, and 400 mg at 0, 2, and 200 mg, 100 mg at 0,
6 weeks 40 mg at 0, 2, and 4 weeks 4 weeks 2 weeks
Maintenance dose 5 mg/kg every 8 weeks 40 mg every 2 weeks 400 mg every 4 weeks 100 mg every 4 weeks
TNF, tumor necrosis factor.
Clinical Uses patients have continued clinical response. Adalimumab and goli-
mumab also are approved for the treatment of moderate to severe
Infliximab, adalimumab, and certolizumab are approved for ulcerative colitis but appear to be less effective than intravenous
the acute and chronic treatment of patients with moderate to infliximab. After induction therapy, less than 55% of patients
severe Crohn’s disease who have had an inadequate response to have a clinical response and less than 20% achieve remission. The
conventional therapies. Infliximab, adalimumab, and golimumab reason why subcutaneous anti-TNF formulations are less effective
are approved for the acute and chronic treatment of moderate to than intravenous infliximab is uncertain.
severe ulcerative colitis. With induction therapy, these approved
agents lead to symptomatic improvement in 60% and disease
remission in 30% of patients with moderate to severe Crohn’s Adverse Effects
disease, including patients who have been dependent on gluco- Serious adverse events occur in up to 6% of patients with anti-
corticoids or who have not responded to 6-MP or methotrex- TNF therapy. The most important adverse effect of these drugs is
ate. The median time to clinical response is 2 weeks. Induction infection due to suppression of the Th1 inflammatory response.
therapy is generally given as follows: infliximab 5 mg/kg intra- This may lead to serious infections such as bacterial sepsis,
venous infusion at 0, 2, and 6 weeks; adalimumab 160 mg (in tuberculosis, invasive fungal organisms, reactivation of hepatitis
divided doses) initially and 80 mg subcutaneous injection at B, listeriosis, and other opportunistic infections. Reactivation
2 weeks; and certolizumab 400 mg subcutaneous injection at 0, 2, of latent tuberculosis, with dissemination, has occurred. Before
and 4 weeks. Patients who respond may be treated with chronic administering anti-TNF therapy, all patients must undergo test-
maintenance therapy, as follows: infliximab 5 mg/kg intravenous ing with tuberculin skin tests or interferon gamma release assays.
infusion every 8 weeks; adalimumab 40 mg subcutaneous injec- Prophylactic therapy for tuberculosis is warranted for patients
tion every 2 weeks; certolizumab 400 mg subcutaneous injection with positive test results before beginning anti-TNF therapy.
every 4 weeks. With chronic, regularly scheduled therapy, clinical More common but usually less serious infections include upper
response is maintained in more than 60% of patients and disease respiratory infections (sinusitis, bronchitis, and pneumonia) and
remission in 40%. However, one-third of patients eventually lose cellulitis. The risk of serious infections is increased markedly in
response despite higher doses or more frequent injections. Loss patients taking concomitant corticosteroids.
of response in many patients may be due to the development of Antibodies to the antibody (ATA) may develop with all four
antibodies to the TNF antibody or to other mechanisms. agents. These antibodies may attenuate or eliminate the clini-
Infliximab is approved for the treatment of patients with mod- cal response and increase the likelihood of developing acute or
erate to severe ulcerative colitis who have had inadequate response delayed infusion or injection reactions. Antibody formation is
to mesalamine or corticosteroids. After induction therapy of much more likely in patients given episodic anti-TNF therapy
5–10 mg/wk at 0, 2, and 6 weeks, 70% of patients have a clinical than regular scheduled injections. In patients on chronic main-
response and one third achieve a clinical remission. With contin- tenance therapy, the prevalence of ATA with infliximab is 10%,
ued maintenance infusions every 8 weeks, approximately 50% of with certolizumab 8%, and with adalimumab or golimumab 3%.