Page 572 - Basic _ Clinical Pharmacology ( PDFDrive )
P. 572
558 SECTION V Drugs That Act in the Central Nervous System
regional analgesic effect while reducing the unwanted respiratory
Somatosensory depression, nausea and vomiting, and sedation that may occur from
cortex the supraspinal actions of systemically administered opioids.
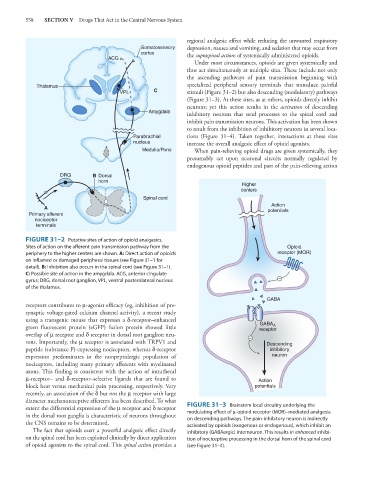
ACG
Under most circumstances, opioids are given systemically and
thus act simultaneously at multiple sites. These include not only
the ascending pathways of pain transmission beginning with
Thalamus specialized peripheral sensory terminals that transduce painful
VPL C stimuli (Figure 31–2) but also descending (modulatory) pathways
(Figure 31–3). At these sites, as at others, opioids directly inhibit
neurons; yet this action results in the activation of descending
Amygdala inhibitory neurons that send processes to the spinal cord and
inhibit pain transmission neurons. This activation has been shown
to result from the inhibition of inhibitory neurons in several loca-
Parabrachial tions (Figure 31–4). Taken together, interactions at these sites
nucleus increase the overall analgesic effect of opioid agonists.
Medulla/Pons When pain-relieving opioid drugs are given systemically, they
presumably act upon neuronal circuits normally regulated by
endogenous opioid peptides and part of the pain-relieving action
DRG B Dorsal
horn
Higher
centers
Spinal cord
Action
A potentials
Primary afferent
nociceptor
terminals
FIGURE 31–2 Putative sites of action of opioid analgesics.
Sites of action on the afferent pain transmission pathway from the Opioid
periphery to the higher centers are shown. A: Direct action of opioids receptor (MOR)
on inflamed or damaged peripheral tissues (see Figure 31–1 for
detail). B: Inhibition also occurs in the spinal cord (see Figure 31–1).
C: Possible site of action in the amygdala. ACG, anterior cingulate
gyrus; DRG, dorsal root ganglion, VPL, ventral posterolateral nucleus
of the thalamus.
GABA
receptors contributes to μ-agonist efficacy (eg, inhibition of pre-
synaptic voltage-gated calcium channel activity), a recent study
using a transgenic mouse that expresses a δ-receptor–enhanced
GABA A
green fluorescent protein (eGFP) fusion protein showed little receptor
overlap of μ receptor and δ receptor in dorsal root ganglion neu-
rons. Importantly, the μ receptor is associated with TRPV1 and Descending
peptide (substance P)-expressing nociceptors, whereas δ-receptor inhibitory
expression predominates in the nonpeptidergic population of neuron
nociceptors, including many primary afferents with myelinated
axons. This finding is consistent with the action of intrathecal
μ-receptor– and δ-receptor–selective ligands that are found to Action
block heat versus mechanical pain processing, respectively. Very potentials
recently, an association of the δ but not the μ receptor with large
diameter mechanoreceptive afferents has been described. To what
extent the differential expression of the μ receptor and δ receptor FIGURE 31–3 Brainstem local circuitry underlying the
modulating effect of μ-opioid receptor (MOR)–mediated analgesia
in the dorsal root ganglia is characteristic of neurons throughout on descending pathways. The pain-inhibitory neuron is indirectly
the CNS remains to be determined. activated by opioids (exogenous or endogenous), which inhibit an
The fact that opioids exert a powerful analgesic effect directly inhibitory (GABAergic) interneuron. This results in enhanced inhibi-
on the spinal cord has been exploited clinically by direct application tion of nociceptive processing in the dorsal horn of the spinal cord
of opioid agonists to the spinal cord. This spinal action provides a (see Figure 31–4).