Page 573 - Basic _ Clinical Pharmacology ( PDFDrive )
P. 573
CHAPTER 31 Opioid Agonists & Antagonists 559
5. Tolerance and dependence—With frequently repeated
therapeutic doses of morphine or its surrogates, there is a gradual
Cortex loss in effectiveness; this loss of effectiveness is termed tolerance.
To reproduce the original response, a larger dose must be admin-
istered. Along with tolerance, physical dependence develops.
Physical dependence is defined as a characteristic withdrawal or
abstinence syndrome when a drug is stopped or an antagonist is
administered (see also Chapter 32).
Midbrain
The mechanism of development of opioid tolerance and physi-
A − Periaqueductal cal dependence is poorly understood, but persistent activation of μ
gray
receptors such as occurs with the treatment of severe chronic pain
appears to play a primary role in its induction and maintenance.
Medulla/pons Current concepts have shifted away from tolerance being driven
by a simple up-regulation of the cyclic adenosine monophosphate
B − Rostral ventral
medulla (cAMP) system. Although this process is associated with toler-
ance, it is not sufficient to explain it. A second hypothesis for the
development of opioid tolerance and dependence is based on the
concept of receptor recycling. Normally, activation of μ receptors
by endogenous ligands results in receptor endocytosis followed
by resensitization and recycling of the receptor to the plasma
Spinal cord membrane (see Chapter 2). However, using genetically modified
C − Dorsal horn mice, research now shows that the failure of morphine to induce
endocytosis of the μ-opioid receptor is an important component
of tolerance and dependence. In further support of this idea,
methadone, a μ-receptor agonist used for the treatment of opioid
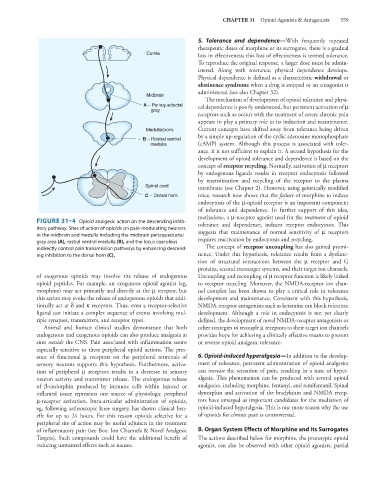
FIGURE 31–4 Opioid analgesic action on the descending inhib- tolerance and dependence, induces receptor endocytosis. This
itory pathway. Sites of action of opioids on pain-modulating neurons
in the midbrain and medulla including the midbrain periaqueductal suggests that maintenance of normal sensitivity of μ receptors
gray area (A), rostral ventral medulla (B), and the locus coeruleus requires reactivation by endocytosis and recycling.
indirectly control pain transmission pathways by enhancing descend- The concept of receptor uncoupling has also gained promi-
ing inhibition to the dorsal horn (C). nence. Under this hypothesis, tolerance results from a dysfunc-
tion of structural interactions between the μ receptor and G
proteins, second-messenger systems, and their target ion channels.
of exogenous opioids may involve the release of endogenous Uncoupling and recoupling of μ receptor function is likely linked
opioid peptides. For example, an exogenous opioid agonist (eg, to receptor recycling. Moreover, the NMDA-receptor ion chan-
morphine) may act primarily and directly at the μ receptor, but nel complex has been shown to play a critical role in tolerance
this action may evoke the release of endogenous opioids that addi- development and maintenance. Consistent with this hypothesis,
tionally act at δ and κ receptors. Thus, even a receptor-selective NMDA-receptor antagonists such as ketamine can block tolerance
ligand can initiate a complex sequence of events involving mul- development. Although a role in endocytosis is not yet clearly
tiple synapses, transmitters, and receptor types. defined, the development of novel NMDA-receptor antagonists or
Animal and human clinical studies demonstrate that both other strategies to recouple μ receptors to their target ion channels
endogenous and exogenous opioids can also produce analgesia at provides hope for achieving a clinically effective means to prevent
sites outside the CNS. Pain associated with inflammation seems or reverse opioid analgesic tolerance.
especially sensitive to these peripheral opioid actions. The pres-
ence of functional μ receptors on the peripheral terminals of 6. Opioid-induced hyperalgesia—In addition to the develop-
sensory neurons supports this hypothesis. Furthermore, activa- ment of tolerance, persistent administration of opioid analgesics
tion of peripheral μ receptors results in a decrease in sensory can increase the sensation of pain, resulting in a state of hyper-
neuron activity and transmitter release. The endogenous release algesia. This phenomenon can be produced with several opioid
of β-endorphin produced by immune cells within injured or analgesics, including morphine, fentanyl, and remifentanil. Spinal
inflamed tissue represents one source of physiologic peripheral dynorphin and activation of the bradykinin and NMDA recep-
μ-receptor activation. Intra-articular administration of opioids, tors have emerged as important candidates for the mediation of
eg, following arthroscopic knee surgery, has shown clinical ben- opioid-induced hyperalgesia. This is one more reason why the use
efit for up to 24 hours. For this reason opioids selective for a of opioids for chronic pain is controversial.
peripheral site of action may be useful adjuncts in the treatment
of inflammatory pain (see Box: Ion Channels & Novel Analgesic B. Organ System Effects of Morphine and Its Surrogates
Targets). Such compounds could have the additional benefit of The actions described below for morphine, the prototypic opioid
reducing unwanted effects such as nausea. agonist, can also be observed with other opioid agonists, partial