Page 10 - Finalll Science BB Prep2 t1 2025_Neat
P. 10
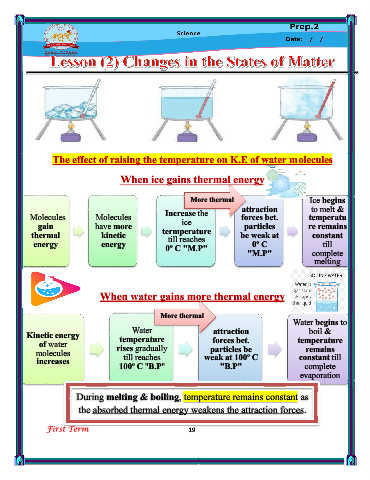
The effect of raising the temperature on K.E of water molecules
When ice gains thermal energy
More thermal Ice begins
attraction
to melt &
energy
Molecules Molecules Increase the forces bet. temperatu
ice
gain have more particles re remains
thermal kinetic termperature be weak at constant
till reaches
energy energy 0º C "M.P" 0º C till
"M.P" complete
melting
When water gains more thermal energy
More thermal
energy Water begins to
Water attraction boil &
Kinetic energy temperature forces bet. temperature
of water rises gradually
particles be
remains
molecules till reaches weak at 100º C constant till
increases 100º C "B.P" "B.P" complete
evaporation
During melting & boiling, temperature remains constant as
the absorbed thermal energy weakens the attraction forces.
First Term 19