Page 78 - Org 3 theoritical book 2024-25
P. 78
Clinical Pharmacy PharmD - 2024/2025 Level 2 Pharmaceutical Organic Chemistry-3 (PC 305)
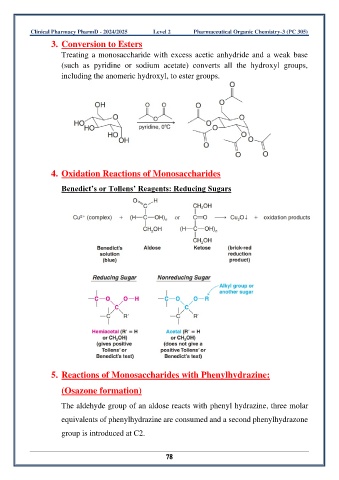
3. Conversion to Esters
Treating a monosaccharide with excess acetic anhydride and a weak base
(such as pyridine or sodium acetate) converts all the hydroxyl groups,
including the anomeric hydroxyl, to ester groups.
4. Oxidation Reactions of Monosaccharides
Benedict’s or Tollens’ Reagents: Reducing Sugars
5. Reactions of Monosaccharides with Phenylhydrazine:
(Osazone formation)
The aldehyde group of an aldose reacts with phenyl hydrazine, three molar
equivalents of phenylhydrazine are consumed and a second phenylhydrazone
group is introduced at C2.