Page 11 - phytochemistry general program
P. 11
but still some alkaloids are neutral (ricinine, colchicines) or amphoteric (morphine
& Narceine). why?
Factors that may influence the degree of basicity:
1- Structure of the molecule such as the degree of unsaturation of the
heterocyclic ring.
Unsaturation decreases the basicity ?? .1
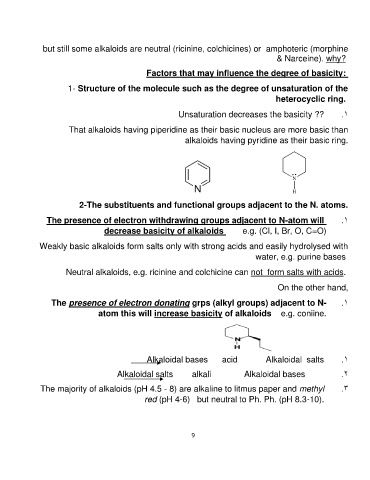
That alkaloids having piperidine as their basic nucleus are more basic than
alkaloids having pyridine as their basic ring.
2-The substituents and functional groups adjacent to the N. atoms.
The presence of electron withdrawing groups adjacent to N-atom will .1
decrease basicity of alkaloids e.g. (Cl, I, Br, O, C=O)
Weakly basic alkaloids form salts only with strong acids and easily hydrolysed with
water, e.g. purine bases
Neutral alkaloids, e.g. ricinine and colchicine can not form salts with acids.
On the other hand,
The presence of electron donating grps (alkyl groups) adjacent to N- .1
atom this will increase basicity of alkaloids e.g. coniine.
Alkaloidal bases acid Alkaloidal salts .1
.2
Alkaloidal salts alkali Alkaloidal bases .3
The majority of alkaloids (pH 4.5 - 8) are alkaline to litmus paper and methyl
red (pH 4-6) but neutral to Ph. Ph. (pH 8.3-10).
9