Page 82 - Phytochemistry 2 (06-PG 605)
P. 82
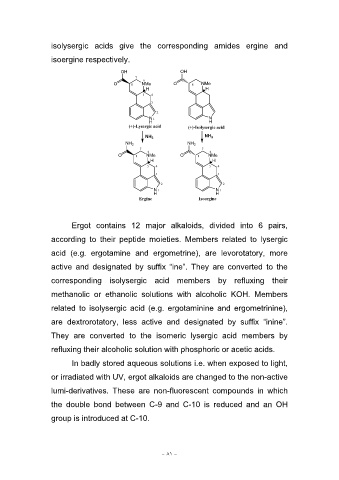
isolysergic acids give the corresponding amides ergine and
isoergine respectively.
OH 6 OH
7 NMe O 8 NMe
H H
O8
54
3
2 N
H
N1 (+)-Isolysergic acid
H
(+)-Lysergic acid
NH3 NH3
NH2 6 NH2 6
7 NMe 7 NMe
H H
O8 O8
54 54
3 3
2 2
N1 N1
H H
Ergine Isoergine
Ergot contains 12 major alkaloids, divided into 6 pairs,
according to their peptide moieties. Members related to lysergic
acid (e.g. ergotamine and ergometrine), are levorotatory, more
active and designated by suffix “ine”. They are converted to the
corresponding isolysergic acid members by refluxing their
methanolic or ethanolic solutions with alcoholic KOH. Members
related to isolysergic acid (e.g. ergotaminine and ergometrinine),
are dextrorotatory, less active and designated by suffix “inine”.
They are converted to the isomeric lysergic acid members by
refluxing their alcoholic solution with phosphoric or acetic acids.
In badly stored aqueous solutions i.e. when exposed to light,
or irradiated with UV, ergot alkaloids are changed to the non-active
lumi-derivatives. These are non-fluorescent compounds in which
the double bond between C-9 and C-10 is reduced and an OH
group is introduced at C-10.
- ۸۱ -