Page 55 - Analytical Chemistry I E-book
P. 55
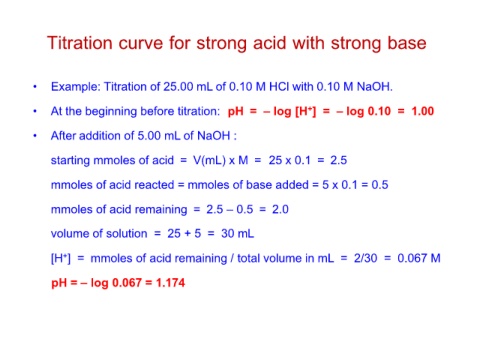
Titration curve for strong acid with strong base
• Example: Titration of 25.00 mL of 0.10 M HCl with 0.10 M NaOH.
• At the beginning before titration: pH = – log [H+] = – log 0.10 = 1.00
• After addition of 5.00 mL of NaOH :
starting mmoles of acid = V(mL) x M = 25 x 0.1 = 2.5
mmoles of acid reacted = mmoles of base added = 5 x 0.1 = 0.5
mmoles of acid remaining = 2.5 – 0.5 = 2.0
volume of solution = 25 + 5 = 30 mL
[H+] = mmoles of acid remaining / total volume in mL = 2/30 = 0.067 M
pH = – log 0.067 = 1.174