Page 51 - Analytical Chemistry I E-book
P. 51
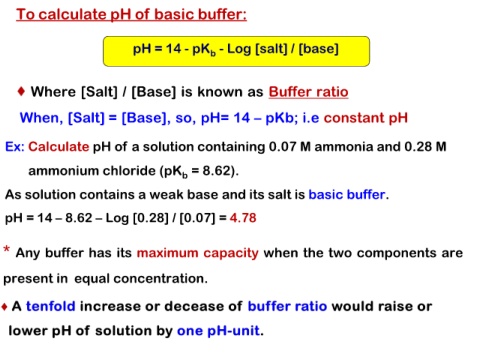
To calculate pH of basic buffer:
pH = 14 - pKb - Log [salt] / [base]
Where [Salt] / [Base] is known as Buffer ratio
When, [Salt] = [Base], so, pH= 14 – pKb; i.e constant pH
Ex: Calculate pH of a solution containing 0.07 M ammonia and 0.28 M
ammonium chloride (pKb = 8.62).
As solution contains a weak base and its salt is basic buffer.
pH = 14 – 8.62 – Log [0.28] / [0.07] = 4.78
* Any buffer has its maximum capacity when the two components are
present in equal concentration.