Page 49 - Analytical Chemistry I E-book
P. 49
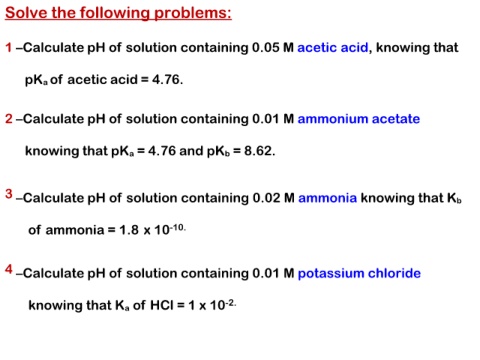
Solve the following problems:
1 –Calculate pH of solution containing 0.05 M acetic acid, knowing that
pKa of acetic acid = 4.76.
2 –Calculate pH of solution containing 0.01 M ammonium acetate
knowing that pKa = 4.76 and pKb = 8.62.
3 –Calculate pH of solution containing 0.02 M ammonia knowing that Kb
of ammonia = 1.8 x 10-10.
4 –Calculate pH of solution containing 0.01 M potassium chloride
knowing that Ka of HCl = 1 x 10-2.