Page 50 - Analytical Chemistry I E-book
P. 50
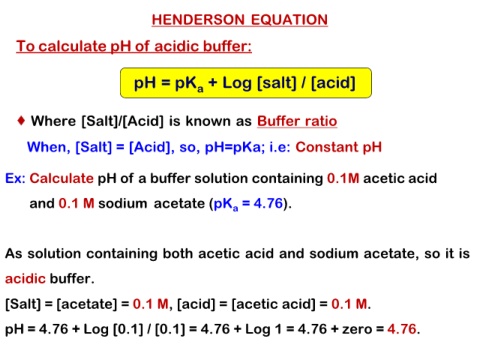
HENDERSON EQUATION
To calculate pH of acidic buffer:
pH = pKa + Log [salt] / [acid]
Where [Salt]/[Acid] is known as Buffer ratio
When, [Salt] = [Acid], so, pH=pKa; i.e: Constant pH
Ex: Calculate pH of a buffer solution containing 0.1M acetic acid
and 0.1 M sodium acetate (pKa = 4.76).
As solution containing both acetic acid and sodium acetate, so it is
acidic buffer.
[Salt] = [acetate] = 0.1 M, [acid] = [acetic acid] = 0.1 M.
pH = 4.76 + Log [0.1] / [0.1] = 4.76 + Log 1 = 4.76 + zero = 4.76.