Page 48 - Analytical Chemistry I E-book
P. 48
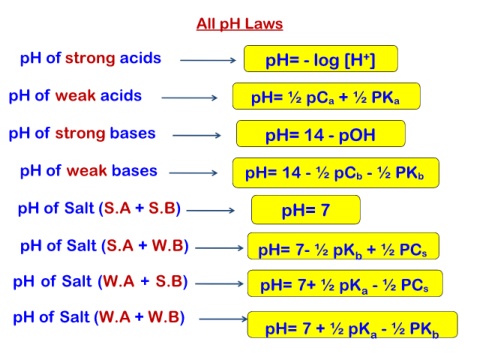
All pH Laws
pH of strong acids pH= - log [H+]
pH of weak acids pH= ½ pCa + ½ PKa
pH of strong bases pH= 14 - pOH
pH of weak bases pH= 14 - ½ pCb - ½ PKb
pH of Salt (S.A + S.B) pH= 7
pH of Salt (S.A + W.B) pH= 7- ½ pKb + ½ PCs
pH of Salt (W.A + S.B) pH= 7+ ½ pKa - ½ PCs
pH of Salt (W.A + W.B)
pH= 7 + ½ pKa - ½ PKb