Page 131 - Pharm.Org.Chem-I 02-06-05-101
P. 131
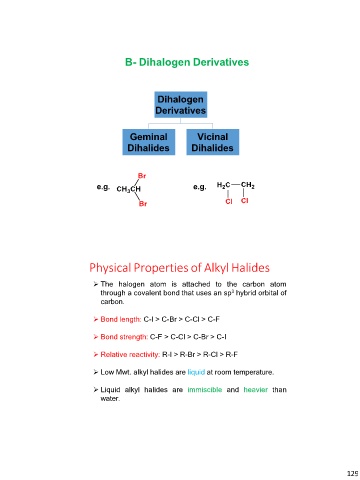
B- Dihalogen Derivatives
Dihalogen
Derivatives
Geminal Vicinal
Dihalides Dihalides
e.g. e.g.
Physical Properties of Alkyl Halides
➢ The halogen atom is attached to the carbon atom
through a covalent bond that uses an sp3 hybrid orbital of
carbon.
➢ Bond length: C-I > C-Br > C-Cl > C-F
➢ Bond strength: C-F > C-Cl > C-Br > C-I
➢ Relative reactivity: R-I > R-Br > R-Cl > R-F
➢ Low Mwt. alkyl halides are liquid at room temperature.
➢ Liquid alkyl halides are immiscible and heavier than
water.
129