Page 142 - Pharm.Org.Chem-I 02-06-05-101
P. 142
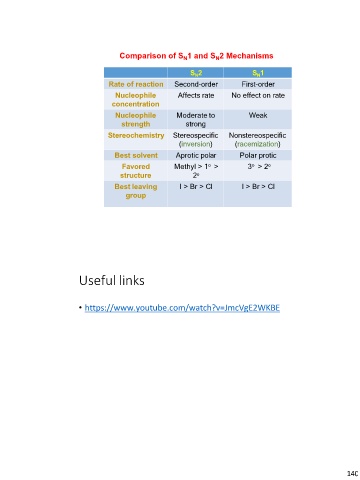
Comparison of SN1 and SN2 Mechanisms
Rate of reaction SN2 SN1
Nucleophile Second-order First-order
concentration Affects rate No effect on rate
Nucleophile
strength Moderate to Weak
strong
Stereochemistry Nonstereospecific
Stereospecific (racemization)
Best solvent (inversion) Polar protic
Favored Aprotic polar 3o > 2o
structure
Methyl > 1o > I > Br > Cl
Best leaving 2o
group
I > Br > Cl
Useful links
• https://www.youtube.com/watch?v=JmcVgE2WKBE
140