Page 144 - Pharm.Org.Chem-I 02-06-05-101
P. 144
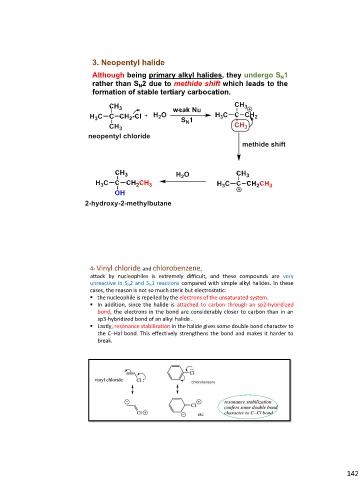
3. Neopentyl halide
Although being primary alkyl halides, they undergo SN1
rather than SN2 due to methide shift which leads to the
formation of stable tertiary carbocation.
4- Vinyl chloride and chlorobenzene,
attack by nucleophiles is extremely difficult, and these compounds are very
unreactive in SN2 and SN1 reactions compared with simple alkyl halides. In these
cases, the reason is not so much steric but electrostatic:
▪ the nucleophile is repelled by the electrons of the unsaturated system.
▪ In addition, since the halide is attached to carbon through an sp2-hybridized
bond, the electrons in the bond are considerably closer to carbon than in an
sp3-hybridized bond of an alkyl halide .
▪ Lastly, resonance stabilization in the halide gives some double bond character to
the C–Hal bond. This effectively strengthens the bond and makes it harder to
break.
142