Page 42 - Pharmaceutical_Analytical_Chemistry_1_Theoretical_Notes_Level_1
P. 42
Mansoura National University
Pharm D-Clinical Pharmacy Program Level 1 Pharm. Anal. Chem. 1 (PC 101)
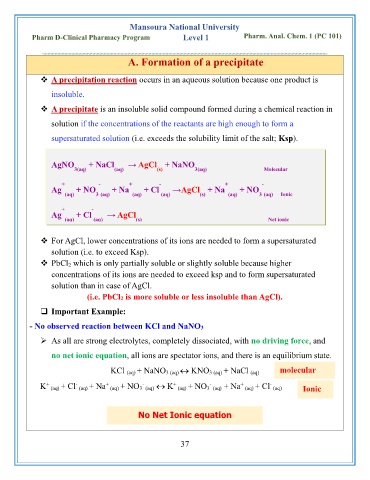
A. Formation of a precipitate
❖ A precipitation reaction occurs in an aqueous solution because one product is
insoluble.
❖ A precipitate is an insoluble solid compound formed during a chemical reaction in
solution if the concentrations of the reactants are high enough to form a
supersaturated solution (i.e. exceeds the solubility limit of the salt; Ksp).
AgNO 3(aq) + NaCl (aq) → AgCl + NaNO 3(aq) Molecular
(s)
+ - + - + -
Ag + NO + Na + Cl →AgCl + Na + NO
(aq) 3 (aq) (aq) (aq) (s) (aq) 3 (aq) Ionic
Ag + + Cl - → AgCl
(aq) (aq) (s) Net ionic
❖ For AgCl, lower concentrations of its ions are needed to form a supersaturated
solution (i.e. to exceed Ksp).
❖ PbCl 2 which is only partially soluble or slightly soluble because higher
concentrations of its ions are needed to exceed ksp and to form supersaturated
solution than in case of AgCl.
(i.e. PbCl2 is more soluble or less insoluble than AgCl).
❑ Important Example:
- No observed reaction between KCl and NaNO3
➢ As all are strong electrolytes, completely dissociated, with no driving force, and
no net ionic equation, all ions are spectator ions, and there is an equilibrium state.
KCl (aq) + NaNO 3 (aq) KNO 3 (aq) + NaCl (aq) molecular
-
-
-
+
+
-
K (aq) + Cl (aq) + Na (aq) + NO 3 (aq) K (aq) + NO 3 (aq) + Na (aq) + Cl (aq) Ionic
+
+
No Net Ionic equation
37