Page 43 - Pharmaceutical_Analytical_Chemistry_1_Theoretical_Notes_Level_1
P. 43
Mansoura National University
Pharm D-Clinical Pharmacy Program Level 1 Pharm. Anal. Chem. 1 (PC 101)
B. Formation of a weak electrolyte
❖ The reaction of sodium acetate and HCl forming acetic acid (vinegar odor) as a
weak electrolyte driving the reaction to right.
HCl (aq) + CH 3COONa (aq) → CH 3COOH (aq) + NaCl (aq) Molecular
-
+
H (aq) + CH 3COO (aq) → CH 3COOH (aq) Net ionic
❖ All neutralization reactions involve the formation of H2O which is a very weak
electrolyte. e.g. reaction of HCl and NaOH
NaOH (aq) + HCl (aq) → NaCl (aq) + H 2O (aq) Molecular
+
-
H + OH → H 2O Net ionic
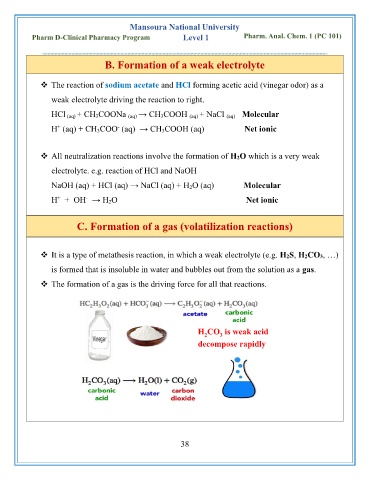
C. Formation of a gas (volatilization reactions)
❖ It is a type of metathesis reaction, in which a weak electrolyte (e.g. H2S, H2CO3, …)
is formed that is insoluble in water and bubbles out from the solution as a gas.
❖ The formation of a gas is the driving force for all that reactions.
H CO is weak acid
2
3
decompose rapidly
38