Page 48 - Pharmaceutical_Analytical_Chemistry_1_Theoretical_Notes_Level_1
P. 48
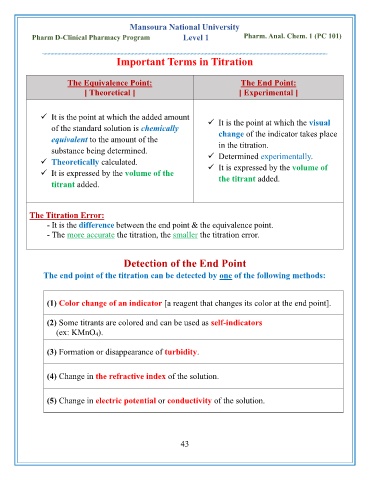
Mansoura National University
Pharm D-Clinical Pharmacy Program Level 1 Pharm. Anal. Chem. 1 (PC 101)
Important Terms in Titration
The Equivalence Point: The End Point:
[ Theoretical ] [ Experimental ]
✓ It is the point at which the added amount ✓ It is the point at which the visual
of the standard solution is chemically change of the indicator takes place
equivalent to the amount of the in the titration.
substance being determined.
✓ Theoretically calculated. ✓ Determined experimentally.
✓ It is expressed by the volume of
✓ It is expressed by the volume of the the titrant added.
titrant added.
The Titration Error:
- It is the difference between the end point & the equivalence point.
- The more accurate the titration, the smaller the titration error.
Detection of the End Point
The end point of the titration can be detected by one of the following methods:
(1) Color change of an indicator [a reagent that changes its color at the end point].
(2) Some titrants are colored and can be used as self-indicators
(ex: KMnO 4).
(3) Formation or disappearance of turbidity.
(4) Change in the refractive index of the solution.
(5) Change in electric potential or conductivity of the solution.
43