Page 30 - Human Umbilical Cord Mesenchymal Stem Cells
P. 30
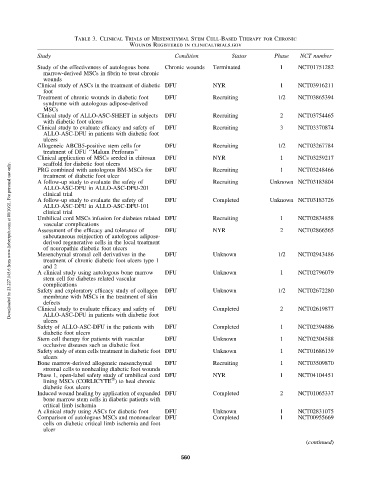
Table 3. Clinical Trials of Mesenchymal Stem Cell-Based Therapy for Chronic
Wounds Registered in clinicaltrials.gov
Study Condition Status Phase NCT number
Study of the effectiveness of autologous bone Chronic wounds Terminated 1 NCT01751282
marrow-derived MSCs in fibrin to treat chronic
wounds
Clinical study of ASCs in the treatment of diabetic DFU NYR 1 NCT03916211
foot
Treatment of chronic wounds in diabetic foot DFU Recruiting 1/2 NCT03865394
syndrome with autologous adipose-derived
MSCs
Clinical study of ALLO-ASC-SHEET in subjects DFU Recruiting 2 NCT03754465
with diabetic foot ulcers
Clinical study to evaluate efficacy and safety of DFU Recruiting 3 NCT03370874
ALLO-ASC-DFU in patients with diabetic foot
ulcers
Allogeneic ABCB5-positive stem cells for DFU Recruiting 1/2 NCT03267784
treatment of DFU ‘‘Malum Perforans’’
Clinical application of MSCs seeded in chitosan DFU NYR 1 NCT03259217
Downloaded by 23.227.145.6 from www.liebertpub.com at 08/10/21. For personal use only.
scaffold for diabetic foot ulcers
PRG combined with autologous BM-MSCs for DFU Recruiting 1 NCT03248466
treatment of diabetic foot ulcer
A follow-up study to evaluate the safety of DFU Recruiting Unknown NCT03183804
ALLO-ASC-DFU in ALLO-ASC-DFU-201
clinical trial
A follow-up study to evaluate the safety of DFU Completed Unknown NCT03183726
ALLO-ASC-DFU in ALLO-ASC-DFU-101
clinical trial
Umbilical cord MSCs infusion for diabetes related DFU Recruiting 1 NCT02834858
vascular complications
Assessment of the efficacy and tolerance of DFU NYR 2 NCT02866565
subcutaneous reinjection of autologous adipose-
derived regenerative cells in the local treatment
of neuropathic diabetic foot ulcers
Mesenchymal stromal cell derivatives in the DFU Unknown 1/2 NCT02943486
treatment of chronic diabetic foot ulcers type 1
and 2
A clinical study using autologous bone marrow DFU Unknown 1 NCT02796079
stem cell for diabetes related vascular
complications
Safety and exploratory efficacy study of collagen DFU Unknown 1/2 NCT02672280
membrane with MSCs in the treatment of skin
defects
Clinical study to evaluate efficacy and safety of DFU Completed 2 NCT02619877
ALLO-ASC-DFU in patients with diabetic foot
ulcers
Safety of ALLO-ASC-DFU in the patients with DFU Completed 1 NCT02394886
diabetic foot ulcers
Stem cell therapy for patients with vascular DFU Unknown 1 NCT02304588
occlusive diseases such as diabetic foot
Safety study of stem cells treatment in diabetic foot DFU Unknown 1 NCT01686139
ulcers
Bone marrow-derived allogeneic mesenchymal DFU Recruiting 1 NCT03509870
stromal cells to nonhealing diabetic foot wounds
Phase 1, open-label safety study of umbilical cord DFU NYR 1 NCT04104451
Ò
lining MSCs (CORLICYTE ) to heal chronic
diabetic foot ulcers
Induced wound healing by application of expanded DFU Completed 2 NCT01065337
bone marrow stem cells in diabetic patients with
critical limb ischemia
A clinical study using ASCs for diabetic foot DFU Unknown 1 NCT02831075
Comparison of autologous MSCs and mononuclear DFU Completed 1 NCT00955669
cells on diabetic critical limb ischemia and foot
ulcer
(continued)
560