Page 573 - The Toxicology of Fishes
P. 573
Chemical Carcinogenesis in Fishes 553
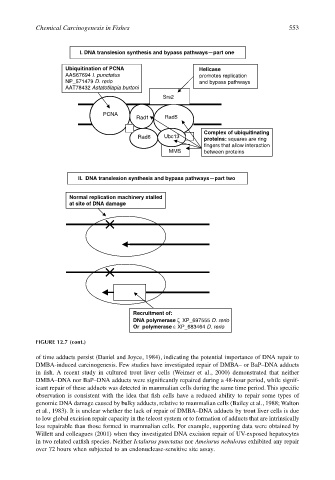
I. DNA translesion synthesis and bypass pathways—part one
Ubiquitination of PCNA Helicase
AAS67694 I. punctatus promotes replication
NP_571479 D. rerio and bypass pathways
AAT78432 Astatotilapia burtoni
Srs2
PCNA
Rad1 Rad5
Complex of ubiquitinating
Rad6 Ubc13 proteins: squares are ring
fingers that allow interaction
MMS between proteins
II. DNA translesion synthesis and bypass pathways—part two
Normal replication machinery stalled
at site of DNA damage
Recruitment of:
DNA polymerase ζ XP_697555 D. rerio
Or polymerase ε XP_683464 D. rerio
FIGURE 12.7 (cont.)
of time adducts persist (Daniel and Joyce, 1984), indicating the potential importance of DNA repair to
DMBA-induced carcinogenesis. Few studies have investigated repair of DMBA– or BaP–DNA adducts
in fish. A recent study in cultured trout liver cells (Weimer et al., 2000) demonstrated that neither
DMBA–DNA nor BaP–DNA adducts were significantly repaired during a 48-hour period, while signif-
icant repair of these adducts was detected in mammalian cells during the same time period. This specific
observation is consistent with the idea that fish cells have a reduced ability to repair some types of
genomic DNA damage caused by bulky adducts, relative to mammalian cells (Bailey et al., 1988; Walton
et al., 1983). It is unclear whether the lack of repair of DMBA–DNA adducts by trout liver cells is due
to low global excision repair capacity in the teleost system or to formation of adducts that are intrinsically
less repairable than those formed in mammalian cells. For example, supporting data were obtained by
Willett and colleagues (2001) when they investigated DNA excision repair of UV-exposed hepatocytes
in two related catfish species. Neither Ictalurus punctatus nor Ameiurus nebulosus exhibited any repair
over 72 hours when subjected to an endonuclease-sensitive site assay.