Page 571 - The Toxicology of Fishes
P. 571
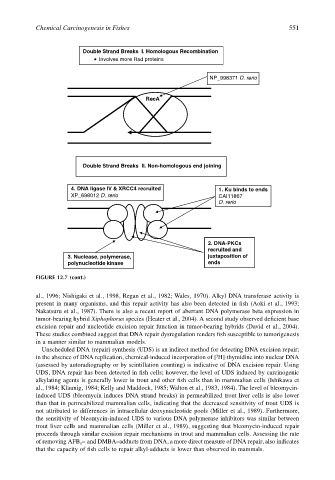
Chemical Carcinogenesis in Fishes 551
Double Strand Breaks I. Homologous Recombination
Involves more Rad proteins
NP_998371 D. rerio
RecA
Double Strand Breaks II. Non-homologous end joining
4. DNA ligase IV & XRCC4 recruited 1. Ku binds to ends
XP_698012 D. rerio CAI11867
D. rerio
2. DNA-PKCs
recruited and
3. Nuclease, polymerase, juxtaposition of
polynucleotide kinase ends
FIGURE 12.7 (cont.)
al., 1996; Nishigaki et al., 1998, Regan et al., 1982; Wales, 1970). Alkyl DNA transferase activity is
present in many organisms, and this repair activity has also been detected in fish (Aoki et al., 1993;
Nakatsuru et al., 1987). There is also a recent report of aberrant DNA polymerase beta expression in
tumor-bearing hybrid Xiphophorus species (Heater et al., 2004). A second study observed deficient base
excision repair and nucleotide excision repair function in tumor-bearing hybrids (David et al., 2004).
These studies combined suggest that DNA repair dysregulation renders fish susceptible to tumorigenesis
in a manner similar to mammalian models.
Unscheduled DNA (repair) synthesis (UDS) is an indirect method for detecting DNA excision repair;
3
in the absence of DNA replication, chemical-induced incorporation of [ H]-thymidine into nuclear DNA
(assessed by autoradiography or by scintillation counting) is indicative of DNA excision repair. Using
UDS, DNA repair has been detected in fish cells; however, the level of UDS induced by carcinogenic
alkylating agents is generally lower in trout and other fish cells than in mammalian cells (Ishikawa et
al., 1984; Klaunig, 1984; Kelly and Maddock, 1985; Walton et al., 1983, 1984). The level of bleomycin-
induced UDS (bleomycin induces DNA strand breaks) in permeabilized trout liver cells is also lower
than that in permeabilized mammalian cells, indicating that the decreased sensitivity of trout UDS is
not attributed to differences in intracellular deoxynucleotide pools (Miller et al., 1989). Furthermore,
the sensitivity of bleomycin-induced UDS to various DNA polymerase inhibitors was similar between
trout liver cells and mammalian cells (Miller et al., 1989), suggesting that bleomycin-induced repair
proceeds through similar excision repair mechanisms in trout and mammalian cells. Assessing the rate
of removing AFB – and DMBA–adducts from DNA, a more direct measure of DNA repair, also indicates
1
that the capacity of fish cells to repair alkyl-adducts is lower than observed in mammals.